Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 10, стр. 1414-1429
Стероидогенный эффект агонистов рецептора лютеинизирующего гормона и метформина у самцов крыс с андрогенным дефицитом, обусловленным диета-индуцированным ожирением
А. А. Бахтюков 1, К. В. Деркач 1, *, И. А. Лебедев 1, В. Н. Сорокоумов 1, 2, А. О. Шпаков 1
1 Институт эволюционной физиологии и биохимии им. И.М. Сеченова РАН
Санкт-Петербург, Россия
2 Институт химии Санкт-Петербургского государственного университета
Санкт-Петербург, Россия
* E-mail: derkatch_k@list.ru
Поступила в редакцию 08.07.2023
После доработки 07.09.2023
Принята к публикации 07.09.2023
- EDN: CLCUZW
- DOI: 10.31857/S0869813923100035
Аннотация
У мужчин с ожирением, наряду с метаболическими нарушениями и инсулиновой резистентностью, снижается уровень тестостерона и нарушаются функции репродуктивной системы. Одним из путей их коррекции может быть применение агонистов рецептора лютеинизирующего гормона (ЛГР) и антидиабетических препаратов, но механизмы их влияния на гипоталамо-гипофизарно-гонадную ось изучены недостаточно. Целью работы было изучить эффекты длительной терапии метформином (5 нед., 120 мг/кг) и пятидневной обработки ЛГР-агонистами – хорионическим гонадотропином человека (ХГЧ, 20 МЕ/крысу/сутки, п/к) и аллостерическим агонистом ТП03 (15 мг/кг/сутки, в/б) на уровень тестостерона в крови и экспрессию тестикулярных и гипофизарных генов у самцов крыс с длительным диета-индуцированным ожирением (ДИО). ТП03 умеренно стимулировал продукцию тестостерона у самцов крыс с ДИО, не оказывая ингибирующего эффекта на экспрессию ЛГР в семенниках и лишь в небольшой степени снижая экспрессию гена β-субъединицы лютеинизирующего гормона в гипофизе. При однократном введении ДИО-крысам стероидогенный эффект ТП03 был сопоставим с таковым в контрольной группе, но при пятидневном введении существенно ему уступал. Стероидогенный эффект ХГЧ при однократном введении крысам с ДИО был ниже такового в контроле, но сопоставим с ним при пятидневном введении ХГЧ, и существенно превосходил соответствующие эффекты ТП03. В отличие от ТП03, ХГЧ значительно снижал экспрессию ЛГР в семенниках и более выражено ингибировал экспрессию лютеинизирующего гормона в гипофизе. Обработка метформином восстанавливала андрогенный статус, существенно не влияя на экспрессию генов стероидогенеза в семенниках. В группах с обработкой метформином не было усиления стероидогенных эффектов обоих ЛГР-агонистов. Полученные результаты указывают на перспективы применения ТП03 и ХГЧ для стимуляции тестикулярного стероидогенеза и на эффективность терапии метформином для нормализации продукции тестостерона при ДИО, что может быть использовано для коррекции репродуктивных расстройств при ожирении. В то же время совместное применение метформина и ЛГР-агонистов при ДИО представляется нецелесообразным.
ВВЕДЕНИЕ
Среди причин нарушений продукции андрогенов и снижения фертильности у мужчин важное место занимают патологическое ожирение и метаболические расстройства, сопровождаемые ожирением, такие как сахарный диабет 2-го типа (СД2), метаболический синдром, стеатоз печени [1–5]. Имеются клинические данные о значимом снижении уровня тестостерона у мужчин с ожирением [1, 2, 4], а также о нарушении функций гипоталамо-гипофизарно-тестикулярной (ГГТ) оси, развитии гипогонадотропных состояний и андрогенном дефиците у подростков пубертатного возраста с явно выраженным ожирением [6, 7]. Получены доказательства негативного влияния диета-индуцированного ожирения (ДИО) на тестикулярный стероидогенез и фертильность у самцов обезьян, крыс и мышей, а также установлены причинно-следственные взаимосвязи между андрогенным дефицитом и ожирением [3, 8, 9]. Среди факторов, провоцирующих андрогенную недостаточность при ожирении, можно выделить инсулиновую и лептиновую резистентность, гипергликемию, дислипидемию, липотоксичность, усиление воспалительных процессов, нарушение окислительно-восстановительного баланса, причем эти факторы могут действовать на различные компоненты ГГТ оси – продуцирующие гонадолиберин гипоталамические нейроны, гонадотрофы аденогипофиза и тестикулярные клетки, в том числе на синтезирующие тестостерон клетки Лейдига [2, 10].
Повышение уровня тестостерона у пациентов с ожирением с помощью заместительной терапии препаратами андрогенов и гонадотропинов, бариатрических операций и низкокалорийной диеты приводит к нормализации массы тела, снижению доли жировой ткани, улучшению липидного метаболизма [5, 11–15]. Однако терапия андрогенами и гонадотропинами имеет ряд побочных эффектов, включая нарушение кроветворения и липидного баланса. Наряду с этим, для нее характерен синдром отмены, когда после прекращения лечения функциональные нарушения в ГГТ оси возвращаются и в ряде случаев даже прогрессируют [16–18]. Возможной альтернативой такой терапии является применение аллостерических регуляторов рецептора лютеинизирующего гормона (ЛГР). В отличие от гонадотропинов – хорионического гонадотропина человека (ХГЧ) и лютеинизирующего гормона (ЛГ), аллостерические регуляторы сравнительно мягко активируют ЛГР, более селективны в отношении внутриклеточных сигнальных каскадов, не вызывают десенситизации ЛГР и резистентности клеток-мишеней к эндогенному ЛГ, как это показано нами ранее для производных тиено[2,3-d]-пиримидина [19–22]. Другим подходом может быть применение препаратов, снижающих массу тела и жировой ткани и улучшающих гормональные и метаболические показатели при ожирении, и здесь наибольший интерес представляет метформин (МФ) – препарат, широко применяемый для лечения СД2 и метаболического синдрома. Имеются данные, что МФ при этих заболеваниях может восстанавливать сперматогенез и андрогенный статус, хотя также получены результаты, которые не поддерживают точку зрения о положительном влиянии МФ на репродуктивные функции при СД2 и метаболическом синдроме [23–26].
Несмотря на имеющийся прогресс в отношении коррекции андрогенного дефицита при метаболических расстройствах, эффективность и механизмы действия гонадотропинов, обладающих ЛГ-активностью, и МФ на стероидогенную активность тестикулярных клеток при ожирении без явных признаков нарушений панкреатических β-клеток остаются малоизученными, а для аллостерических ЛГР-агонистов такие данные вовсе отсутствуют. Нет сведений о влиянии комбинированного применения МФ и ЛГР-агонистов на продукцию тестостерона при ожирении. Влияние аллостерических ЛГР-агонистов на экспрессию гипофизарных генов, кодирующих компоненты системы синтеза гонадотропинов, до настоящего времени не исследовано, а для диета-индуцированного ожирения (ДИО) такие сведения отсутствуют и для ХГЧ, и для МФ. Поскольку ожирение тесно связано с андрогенным дефицитом и гипогонадотропными состояниями, то такие исследования имеют несомненное практическое значение. Целью работы было изучить эффекты длительной (5 нед.) терапии МФ и пятидневной обработки ЛГР-агонистами, ХГЧ и разработанным нами тиено[2,3-d]-пиримидиновым производным ТП03 на уровень тестостерона в крови и на экспрессию тестикулярных генов, ответственных за стероидогенез, и гипофизарных генов, вовлеченных в синтез и секрецию гонадотропинов, у самцов крыс с ожирением, вызванным длительной высокожировой диетой. Наряду с раздельным применением препаратов, исследовали введение ЛГР-агонистов на фоне МФ терапии, чтобы проверить возможность потенцирующего влияния МФ на их эффекты. Ранее нами было продемонстрировано усиление стероидогенных эффектов ЛГР-агонистов при их однократном введении самцам крыс с СД2, длительное время леченных МФ [21]. Выбор ТП03 обусловлен его отчетливо выраженной стероидогенной активностью, показанной нами ранее у контрольных, диабетических и стареющих самцов крыс [20].
МЕТОДЫ ИССЛЕДОВАНИЯ
В экспериментах использовали самцов крыс линии Wistar, которым в начале эксперимента было два месяца. Ожирение и ассоциированные с ним метаболические и гормональные расстройства вызывали переводом двухмесячных самцов крыс на высокожировую диету, которая включала 52.4% жира (свиного сала), 41.7% творога, 5% паштета (свиная печень), 0.5% метионина, 0.2% дрожжей и 0.2% поваренной соли (NaCl) [27]. Крысы на протяжении 23 нед. получали ежедневно по 5–7 г высокожировой смеси, дополнительно к стандартному рациону (гранулированный сухой корм). Контрольная группа животных того же возраста получала только гранулированный сухой корм.
Перед началом лечения отбирали крыс с повышенным содержанием глюкозы в крови (выше 7.0 мМ) через 120 мин после глюкозной нагрузки в интраперитонеальном глюкозотолерантном тесте (ИГТТ, 2 г глюкозы/животное, в/б), а также с повышенной массой тела, рассматривая их как группу с ожирением и нарушенной толерантностью к глюкозе. Затем крыс с ожирением случайным образом распределяли на экспериментальные группы (в каждой n = 18), одна из которых (группа “ОЖ + МФ”) в течение 5 нед. получала МФ (“Sigma”, США; 120 мг/кг/сутки, перорально через зонд), другая (группа “ОЖ”) вместо МФ получала плацебо (водный раствор). Обе группы до конца эксперимента продолжали получать пищу с высоким содержанием насыщенных жиров. За неделю до окончания эксперимента, перед обработкой животных ЛГР-агонистами, проводили повторный ИГТТ для изучения чувствительности к глюкозе и инсулинового и лептинового ответа на глюкозную нагрузку, оценивая уровни глюкозы, инсулина и лептина в крови через различные временные интервалы после введения глюкозы. Для определения уровня глюкозы использовали глюкометр и тест-полоски “One-Touch Select Ultra” (США), для определения концентрации инсулина и лептина – наборы “Rat Insulin ELISA” (“Mercodia AB”, Швеция) и “ELISA Kit for Leptin” (Cloud-Clone Corp., США). В крови животных оценивали уровни гликированного гемоглобина (HbA1c), общего холестерина и триглицеридов, для чего использовали набор “Multi Test HbA1c System kit” (“Polymer Technology Systems, Inc.”, США) и тест-полоски “Cholesterol multiCare-in” и “Triglycerides multiCare-in” (“Biochemical Systems Int.”, Италия). На следующий день после ИГТТ оценивали уровни тестостерона в крови животных на протяжении шестичасового временного промежутка (в 10.00, 12.00, 14.00 и 16.00), рассчитывая интегративное значение AUC0–6 (в относительных единицах). Концентрацию тестостерона определяли с помощью набора “Тестостерон-ИФА” (“Алкор-Био”, Россия). Образцы крови забирали из хвостовой вены под местной анестезией, которую осуществляли с помощью 2%-ного раствора лидокаина из расчета 2–4 мг/кг массы тела.
После повторного тестирования группы “ОЖ” и “ОЖ + МФ” рандомизировали каждую на три подгруппы, после чего животных в течение 5 дней обрабатывали ЛГР-агонистами (ТП03, ХГЧ) или растворителем (ДМСО, растворитель для ТП03). Ранее было показано, что в используемом объеме ДМСО не влияет на метаболические и гормональные показатели [20]. Синтез и характеристику ТП03 осуществляли, как описано нами ранее [21], в то время как ХГЧ был производства “Московского эндокринного завода” (Россия).
Тем самым были сформированы следующие группы (в каждой n = 6): “К” (контроль), “ОЖ” (без лечения), “ОЖ + ТП” (ТП03, 15 мг/кг/сутки, в/б, 5 дней), “ОЖ + Г” (ХГЧ, 20 МЕ/крысу/сутки, п/к, 5 дней), “ОЖМ” (МФ, 120 мг/кг/сутки, перорально), “ОЖМ + ТП” (совместно МФ и ТП03), “ОЖМ + Г” (совместно МФ и ХГЧ). В ходе эксперимента оценивали концентрацию тестостерона в крови крыс после однократного введения ЛГР-агонистов (1-й день, забор крови до введения, которое осуществляли в 10.00, и через 1, 3 и 5 ч после него) и ежедневно в течение пяти дней через 3 ч после их введения (13.00). Сразу после последнего забора крови животных наркотизировали (хлоральгидрат, 400 мг/кг), декапитировали и забирали у них ткани семенников и гипофиза для оценки экспрессии целевых генов, а также оценивали массу адбоминального жира и рассчитывали его долю в общей массе тела.
Количественную ПЦР в реальном времени осуществляли, как описано ранее [20], для чего из семенников и гипофиза выделяли тотальную РНК, используя “ExtraRNA kit” (“Evrogen”, Россия). Обратную транскрипцию проводили, используя набор “MMLV RT Kit” (“Evrogen”, Россия). Амплификационный сигнал детектировали, используя прибор “7500 Real-Time PCR System” (“Thermo Fisher Scientific Inc.”, США). Для исследований использовали последовательности комплементарных кДНК прямого и обратного праймеров для генов крысы, кодирующих ЛГР (Lhr) и стероидогенные белки – холестерин-транспортирующий белок StAR (Star), цитохромы CYP11A1 (Cyp11a1), CYP17A1 (Cyp17a1) и CYP19A1 (Cyp19a1) в семенниках, и гены, кодирующие рецептор гонадолиберина (Gnrhr), β-субъединицы ЛГ (LHbeta) и ФСГ (FSHbeta) в гипофизе (табл. 1). В качестве референсных использовали гены 18S-рРНК (18S rRNA) и β-актина (Actb). Анализ результатов проводили с использованием метода ΔΔСt. Значения RQ рассчитывали по отношению к контрольной группе, в которой их принимали за единицу.
Таблица 1.
Структура праймеров, использованных для оценки экспрессии целевых генов в семенниках и гипофизе, а также референсных генов Actb и 18S rRNA
| Ген | Последовательность праймеров | Т отжига, °С | NCBI Reference Sequence |
|---|---|---|---|
| Lhr | For: CTGCGCTGTCCTGGCC Rev: CGACCTCATTAAGTCCCCTGAA |
55 | NM_012978.1 |
| Star | For: AAGGCTGGAAGAAGGAAAGC Rev: CACCTGGCACCACCTTACTT |
55 | NM_031558.3 |
| Cyp11a1 | For: TATTCCGCTTTGCCTTTGAG Rev: CACGATCTCCTCCAACATCC |
55 | NM_017286.3 |
| Cyp17a1 | For: CATCCCCCACAAGGCTAAC Rev: TGTGTCCTTGGGGACAGTAAA |
55 | XM_006231435.3 |
| Cyp19a1 | For: GGTATCAGCCTGTCGTGGAC Rev: AGCCTGTGCATTCTTCCGAT |
56 | NM_017085.2 |
| Gnrhr | For: TGGGAGACTACCCAAGCACT Rev: TGCTAACCTGAGCCCAAACC |
56 | NM_031038.3 |
| LHbeta | For: TCACCTTCACCACCAGCATC Rev: GGTAGGTGCACACTGGCTGA |
56 | NM_012858.2 |
| FSHbeta | For: TCGATCCAGCTTTGCATCCT Rev: TGGTGTAGCAGTAGCCCTCA |
56 | NM_001007597.2 |
| Actb | For: CTGGCACCACACCTTCTACA Rev: AGGTCTCAAACATGATCTGGGT |
56 | NM_031144.3 |
| 18S rRNA | For: GGACACGGACAGGATTGACA Rev: ACCCACGGAATCGAGAAAGA |
56 | NR_046237.1 |
Lhr – ген рецептора лютеинизирующего гормона; Star – ген холестерин-транспортирующего белка StAR (steroidogenic acute regulatory protein); Cyp11a1 – ген цитохрома CYP11A1 (P450scc), катализирующего превращение холестерина в прегненолон; Cyp17a1 – ген цитохрома CYP17A1 (P450 17A1 17α-гидроксилаза/17,20-лиаза), катализирующего превращение прогестерона в 17-гидроксипрогестерон и далее в андростендион; Cyp19a1 – ген цитохрома CYP19A1 (ароматазы), катализирующего конверсию андрогенов в эстрогены путем ароматизации кольца А, что обеспечивает превращение андростендиона в эстрон и тестостерона в эстрадиол; Gnrhr – ген рецептора гонадолиберина, относящегося к G-белок-сопряженным рецепторам; LHbeta и FSHbeta – гены β-субъединиц ЛГ и фолликулостимулирующего гормона (ФСГ) соответственно, определяющие типовую принадлежность гонадотропина.
Статистический анализ проводили с помощью пакета программ “SPSS Statistics”, используя однофакторный дисперсионный анализ с попарным сравнением с помощью апостериорного критерия Бонферрони. Статистически значимыми считали различия при уровне значимости p < 0.05.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Масса тела и абдоминального жира, а также содержание в крови триглицеридов и общего холестерина у крыс с ожирением значимо превышали эти показатели в контрольной группе (табл. 2). В группе “ОЖ” отмечали повышенные уровни глюкозы, инсулина и лептина, как базальные, так и через 120 мин после глюкозной нагрузки, повышенное содержание гликированного гемоглобина, а также нарушенную толерантность к глюкозе, о чем свидетельствует повышенное значение AUC0–120 для глюкозной кривой в ИГТТ (табл. 2). Лечение МФ приводило к нормализации массы тела, базального и стимулированного глюкозной нагрузкой уровней инсулина и лептина, значимо снижало массу жировой ткани и содержание HbA1c, восстанавливало глюкозную чувствительность и липидный профиль (табл. 2). В группе “ОЖ” базовая концентрация тестостерона в крови была снижена. Лечение МФ повышало ее, на что указывает рассчитанное значение AUC0–6(часы) для тестостерона в 6-часовом временном промежутке (табл. 2). Тем самым четырехнедельное лечение МФ частично восстанавливало метаболические и гормональные показатели у крыс с ДИО, включая их андрогенный статус.
Таблица 2.
Масса тела и жировой ткани, липидный профиль, уровни глюкозы, инсулина и лептина при проведении теста с глюкозной нагрузкой и уровни тестостерона у самцов крыс с ДИО, в том числе леченных метформином, в сравнении с контрольными животными
| Контроль | ОЖ | ОЖ + МФ | |
|---|---|---|---|
| Масса тела, г | 403.6 ± 7.7 | 446.3 ± 7.6a | 424.6 ± 6.5 |
| Масса жира, г | 7.14 ± 0.23 | 9.97 ± 0.50a | 8.46 ± 0.30a,b |
| Доля жира, % | 1.76 ± 0.03 | 2.22 ± 0.07a | 1.98 ± 0.04a,b |
| Глюкоза (0), мМ | 5.8 ± 0.1 | 6.5 ± 0.2a | 6.0 ± 0.2 |
| Глюкоза (120), мМ | 6.2 ± 0.2 | 9.0 ± 0.3a | 8.0 ± 0.3a,b |
| AUC0–120(мин) (глюкоза), усл. ед. | 1188 ± 52 | 1811 ± 44a | 1563 ± 44a,b |
| Инсулин (0), нг/мл | 0.94 ± 0.15 | 1.55 ± 0.15a | 1.32 ± 0.19 |
| Инсулин (60), нг/мл | 2.16 ± 0.19 | 4.76 ± 0.43a | 2.78 ± 0.29b |
| Инсулин (120), нг/мл | 1.20 ± 0.22 | 2.75 ± 0.51a | 1.43 ± 0.09 |
| Лептин (0), нг/мл | 1.64 ± 0.23 | 2.34 ± 0.37a | 1.86 ± 0.62 |
| Лептин (120), нг/мл | 4.22 ± 0.90 | 11.97 ± 1.62a | 6.96 ± 1.24 |
| HbA1c, % | 4.12 ± 0.06 | 4.85 ± 0.06a | 4.43 ± 0.07b |
| Триглицериды, мМ | 2.06 ± 0.07 | 3.19 ± 0.14a | 2.70 ± 0.11a,b |
| Холестерин, мМ | 4.32 ± 0.11 | 6.81 ± 0.16a | 6.04 ± 0.16a,b |
| Тестостерон (10.00), нМ | 8.43 ± 0.67 | 4.51 ± 0.39a | 5.47 ± 0.29a |
| Тестостерон (16.00), нМ | 9.25 ± 0.56 | 5.52 ± 0.40a | 7.11 ± 0.48a,b |
| AUC0–6(часы) (тестостерон), усл. ед. | 51.4 ± 2.6 | 30.8 ± 2.1a | 41.0 ± 2.1a,b |
Различия по сравнению с группами “К” (a) и “ОЖ” (b) статистически значимы при p < 0.05. Значения AUC0–120(мин) – интегрированная площадь под кривой “концентрация глюкозы (мМ)–время (мин)”, включающая временной промежуток 120 мин от момента в/б инъекции глюкозы (2 г/кг) с пятью измерениями уровня глюкозы в крови (до и через 15, 30, 60 и 120 мин после глюкозной нагрузки). Значения AUC0–6(часы) – интегрированная площадь под кривой “концентрация тестостерона (нМ)–время (часы)”, включающая временной промежуток от 10.00 до 16.00 (четыре измерения – в 10.00, 12.00, 14.00 и 16.00). Данные представлены как M ± SEM, во всех группах n = 18.
Однократная обработка крыс с ожирением с помощью ТП03 и ХГЧ, как и в случае контрольных животных, приводила к стимуляции продукции тестостерона (рис. 1), о чем свидетельствуют значения AUC1–5(часы) (табл. 3). При этом приросты уровня тестостерона в группе “ОЖ” с обработкой ХГЧ были существенно ниже, чем в контроле, составляя через 3 и 5 ч после начала обработки 58 и 65% от таковых в группе “К + Г”, о чем также свидетельствует снижение на 39% значения AUC1–5(часы) в группе “ОЖ + Г” в сравнении с группой “К + Г” (рис. 1a и 1b, табл. 3). Стероидогенные эффекты ТП03 были выражены слабее, чем в случае гонадотропина, и в группах “К + ТП” и “ОЖ + ТП” были сопоставимыми (рис. 1a и 1b, табл. 3). Лечение МФ, улучшая базовый андрогенный статус, не приводило к восстановлению стероидогенных эффектов ЛГР-агонистов (рис. 1c, табл. 3).
Рис. 1.
Уровни тестостерона в сыворотке крови в течение 5 ч после введения ТП03 и ХГЧ самцам крыс с ожирением, в том числе леченных метформином, в сравнении с таковыми в контрольной группе. Обозначения групп: (a): С (К) – контроль; C + TP (К + ТП) – ТП03, 15 мг/кг/сутки, в/б, 5 дней; C + + G (К + Г) – ХГЧ, 20 МЕ/крысу/сутки, п/к, 5 дней; (b): OB (ОЖ) – крысы с ожирением без лечения; OB +TP (ОЖ + ТП) – крысы с ожирением, с обработкой ТП03, 15 мг/кг/сутки, в/б, 5 дней; OB + + G (ОЖ + Г) – крысы с ожирением, с обработкой ХГЧ, 20 МЕ/крысу/сутки, п/к, 5 дней; (c): OBM (ОЖМ) – крысы с ожирением, обработанные метформином, 120 мг/кг/сутки, перорально, 5 недель; OBM + TP (ОЖМ + ТП) – крысы с ожирением, с обработкой метформином и ТП03; OBM + G (ОЖМ + Г) – крысы с ожирением, с обработкой метформином и ХГЧ.
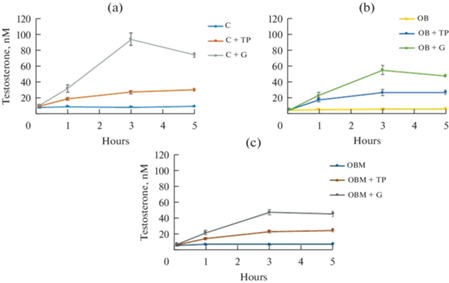
Таблица 3.
Значения интегрированной площади под кривой “концентрация тестостерона (нМ)–время (минуты/дни)” (AUC1–5(часы)/AUC1–5(дни)) при однократном или пятидневном введении ТП03 и ХГЧ контрольным крысам и животным с ожирением, в том числе леченным метформином (в усл. ед.)
| Группа | AUC1–5(часы), усл. ед. | AUC1–5(дни), усл. ед. |
|---|---|---|
| К | 39.0 ± 2.6 | 37.5 ± 3.6 |
| К + ТП | 106.9 ± 7.1a | 136.9 ± 15.8a |
| К + Г | 296.2 ± 17.0a,d | 217.6 ± 9.2a |
| ОЖ | 23.0 ± 2.4a | 19.3 ± 2.5a |
| ОЖ + ТП | 97.8 ± 11.2a,b | 88.6 ± 7.3a,b,c |
| ОЖ + Г | 181.7 ± 14.8a,b,c,d | 171.1 ± 17.5a,b,d |
| ОЖМ | 30.7 ± 2.6 | 28.3 ± 2.8 |
| ОЖМ + ТП | 88.2 ± 6.1a,b | 69.0 ± 8.0a,b,c |
| ОЖМ + Г | 166.1 ± 11.9a,b,c,d | 213.7 ± 18.8a,b,d |
Значения AUC1–5(часы) и AUC1–5(дни) – интегрированная площадь под кривой “концентрация тестостерона (нМ)–время (часы/дни)”, включающая временной промежуток от 11.00 до 15.00 (три измерения – в 11.00, 13.00 и 15.00, введение препаратов – 10.00) или с первого по пятый дни введения (ежедневно в 13.00 через 3 ч после введения препаратов). Различия по сравнению с группами “К” (a) и “ОЖ” (b) статистически значимы при p < 0.05; различия между контрольными и ОЖ/ОЖМ крысами с обработкой ТП03 или ХГЧ (“К + ТП”/“ОЖ + ТП”, “К + ТП"/"ОЖМ + ТП”, “К + Г”/“ОЖ + Г”, “К +Г”/“ОЖМ + Г”) (c) и с обработкой ТП03 и ХГЧ (“К + ТП”/“К + Г”; “ОЖ + ТП”/“ОЖ + Г”; “ОЖМ + ТП”/“ОЖМ + Г”) (d) статистически значимы при p < 0.05. Различия между группами с лечением и без лечения МФ (“ОЖ + + ТП”/“ОЖМ + ТП”; “ОЖ + Г”/“ОЖМ + Г”) ни в одном случае не были значимыми (p > 0.05). Данные представлены как M ± SEM, во всех группах n = 6.
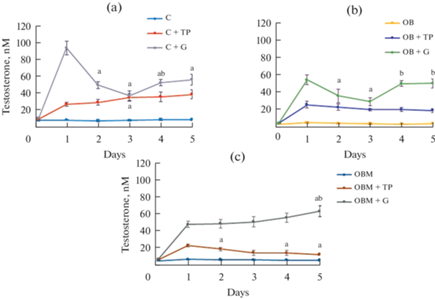
Пятидневная обработка с помощью ЛГР-агонистов во всех изученных группах приводила к повышению уровня тестостерона в крови, но стероидогенные эффекты существенно различались как по величине, так и по динамике (рис. 2). В контрольной группе стимулирующие продукцию тестостерона эффекты ТП03 составляли 176% в первый день обработки и постепенно повышались, достигая 289% на 5-й день. В группе “ОЖ+ТП” стимулирующий эффект ТП03 существенно не менялся в течение 5-дневной обработки препаратом, а в группе “ОЖМ+ТП” эффект ТП03 снижался и на 5-й день обработки составлял 55% от стимулирующего эффекта, полученного в 1-й день обработки (рис. 2b и 2c, табл. 3). Стероидогенные эффекты ХГЧ в группах “К + Г” и “ОЖ + Г” были максимальны в первый день обработки (726 и 978% соответственно), затем на 3-й день достоверно снижались на 60 и 46% соответственно, после чего восстанавливались, и на 4-й день в группе “К + Г” были в 1.4 раза выше, а в группе “ОЖ + Г” в 1.7 раза выше, чем на 3-й день обработки. При этом в 4–5-й дни в контрольной группе они были ниже, а в группе с ожирением сходными с таковыми в первый день обработки (рис. 2a и 2b). Важно отметить, что в группе “ОЖМ + Г” снижение эффекта гонадотропина в 2–4-й дни отсутствовало, а на пятый день обработки ХГЧ содержание тестостерона в крови нарастало и было достоверно выше, чем в первый день (рис. 2c). Тем самым при ожирении динамика стероидогенных эффектов ТП03 и ХГЧ не претерпевало значимых изменений, а в группах с лечением МФ отмечалось ослабление стероидогенного эффекта ТП03 и изменение динамики соответствующего эффекта ХГЧ (рис. 2, табл. 3). Оценка интегрированных значений уровней тестостерона показала, что, в отличие от однократного введения, значение AUC1–5(дни) для ХГЧ в группах “ОЖ + Г” и “ОЖМ + Г” значимо не отличались от такового в группе “К + Г”, что указывает на сохранение продукции тестостерона при длительном введении гонадотропина, в то время как в случае ТП03, напротив, начинало выявляться снижение значений AUC1–5(дни), в наибольшей степени в группе “ОЖМ + ТП” (табл. 3).
Рис. 2.
Уровни тестостерона в сыворотке крови в течение пятидневного введения ТП03 и ХГЧ самцам крыс с ожирением (b), в том числе леченных метформином (c), в сравнении с таковыми в контрольной группе (c). Различия по сравнению с первым днем введения (a) и с третьим днем введения препаратов (b) статистически значимы при p < 0.05. Обозначения групп как на рис. 1.
Анализ экспрессии тестикулярных генов показал, что в группе “ОЖ” снижена экспрессия гена Star, причем обработка МФ лишь частично восстанавливала его экспрессию (рис. 3). Пятидневная обработка контрольных крыс с помощью ТП03 существенно не влияла на экспрессию изученных стероидогенных генов, в то время как обработка ХГЧ в значительной степени снижала экспрессию гена Lhr и в среднем в два раза повышала экспрессию стероидогенных генов Star, Cyp11a1 и Cyp17a1 (рис. 3).
Рис. 3.
Экспрессия генов, кодирующих ЛГР и стероидогенные белки, в семенниках крыс с ожирением, в том числе леченных метформином, после введения им ТП03 и ХГЧ, в сравнении с контрольной группой. Обозначения групп как на рис. 1. Различия по сравнению с группами “К” (a) и “ОЖ” (b) статистически значимы при p < 0.05; различия между группами “К + Г” и “ОЖМ + Г” (c), между группами с обработкой ТП03 и ХГЧ (“К + ТП”/“К + Г”; “ОЖ + ТП”/“ОЖ + Г”; “ОЖМ + ТП”/”ОЖМ + Г”) (d) или между группами с лечением и без лечения МФ (“ОЖ + ТП”/“ОЖМ + ТП”; “ОЖ + Г”/“ОЖМ + Г”) (e) статистически значимы при p < 0.05. Данные представлены как M ± SEM, во всех группах n = 6.
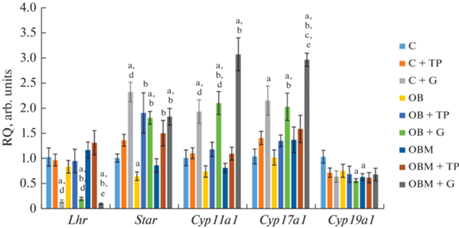
В группах с ожирением ТП03 повышал экспрессию гена Star в сравнении с группой “ОЖ” (но не с контролем), не влияя на экспрессию гена Lhr (рис. 3). Стимулирующие эффекты ХГЧ на экспрессию генов Star, Cyp11a1 и Cyp17a1 в группах “ОЖ + Г” и “ОЖМ + Г” сохранялись, причем обработка МФ усиливала эффект ХГЧ в отношении гена Cyp17a1. В то же время отмечалось значительное ингибирование экспрессии гена Lhr (рис. 3). Оба ЛГР-агониста существенно не влияли на экспрессию гена ароматазы, которая во всех группах была близка к таковой в контроле. Тем самым ТП03 повышал экспрессию гена Star, но слабо влиял на экспрессию генов, кодирующих ЛГР и другие стероидогенные белки. В то же время ХГЧ значительно (на 80–89%) подавлял экспрессию гена Lhr, но усиливал экспрессию генов стероидогенных белков, причем величина его эффекта у крыс с ожирением, в том числе леченных МФ, не уступала таковой в контроле, и эти данные согласуются со стероидогенными эффектами ЛГР-агонистов.
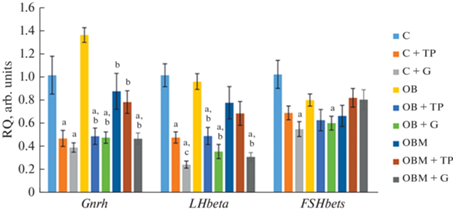
На заключительном этапе исследовали экспрессию гипофизарных генов, кодирующих рецептор гонадолиберина, через который этот рилизинг-фактор осуществляет стимуляцию секреции и экспрессии гонадотропинов в гонадотрофах, а также β-субъединиц ЛГ и ФСГ, которые определяют специфичность гетеродимерных гонадотропинов, поскольку их α-субъединицы по первичной структуре идентичны. При ожирении и лечении крыс с ожирением МФ экспрессия изученных гипофизарных генов существенно не менялась по сравнению с контролем, но обработка МФ значимо снижала экспрессию гена Gnrhr в сравнении с группой ОЖ (рис. 4). ТП03 и ХГЧ снижали экспрессию генов Gnrhr и LHbeta, причем в случае гена LHbeta различия между контролем и группами “К + ТП” и “К + Г” были значимыми. В свою очередь, ингибирование экспрессии гена FSHbeta было показано только в случае воздействия ХГЧ на группы “К + Г” и “ОЖ + Г” (рис. 4).
Рис. 4.
Экспрессия генов гонадотропинов и рецептора гонадолиберина в гипофизе самцов крыс с ожирением, в том числе леченных метформином, после введения им ТП03 и ХГЧ, в сравнении с таковой в контрольной группе животных. Обозначения групп как на рис. 1. Различия по сравнению с группами “К” (a) и “ОЖ” (b) статистически значимы при p < 0.05; различия между группами “К + ТП” и “К + Г” (c) статистически значимы при p < 0.05. Данные представлены как M ± SEM, во всех группах n = 6.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
У мужчин патологическое ожирение и другие метаболические расстройства, обусловленные нарушениями углеводного и липидного обмена, нарушенной толерантностью к глюкозе и инсулиновой резистентностью, ассоциированы с андрогенным дефицитом и репродуктивными дисфункциями, что требует разработки эффективных подходов для нормализации продукции тестостерона и восстановления репродуктивного потенциала [1–7]. Такие подходы могут быть использованы превентивно, на ранних стадиях нарушений метаболического и гормонального статуса, для предотвращения тяжелых форм ожирения, метаболического синдрома и СД2, поскольку андрогенная недостаточность может быть не только следствием, но и триггером этих эндокринных расстройств [3, 8, 28]. В экспериментальных условиях для изучения нарушений тестикулярной функции при ожирении и поиска путей их восстановления наиболее подходящей является модель ДИО у грызунов, в том числе у крыс. Самцы крыс с ДИО имеют сниженный уровень тестостерона и нарушенный сперматогенез [3, 8, 29]. Эти данные в целом согласуются с нашими результатами, полученными на модели ожирения у самцов крыс, вызванного длительной (23 нед.) диетой, обогащенной насыщенными животными жирами. Нами продемонстрировано снижение у крыс с ожирением базового уровня тестостерона в крови и экспрессии белка StAR, катализирующего первую, скорость-лимитирующую стадию стероидогенеза в семенниках, а также показано значимое ослабление стероидогенного эффекта ХГЧ при его однократном введении животным. В то же время в семенниках крыс с ожирением нами не было показано значимого изменения экспрессии генов, кодирующих ключевые стероидогенные ферменты – цитохромы CYP11A1 и CYP17A1 и фермент ароматазу (CYP19A1), осуществляющую конверсию андрогенов в эстрогены. Другие авторы на основе анализа транскриптома в семенниках андроген-дефицитных крыс Wistar с ожирением, индуцированным восьминедельной высокожировой диетой, показали повышение экспрессии гена ароматазы, результатом чего было снижение соотношения тестостерон/эстрадиол в семенниках и ухудшение сперматогенеза [30]. В этой связи следует отметить, что продолжительность ожирения, как и особенности диеты, существенно влияют на выраженность изменений андрогенного статуса и на паттерн экспрессии компонентов системы тестикулярного стероидогенеза. В нашем случае продолжительность диеты составила 23 нед., вследствие чего, как мы полагаем, длительное, хотя и умеренное снижение продукции тестостерона могло компенсаторно снизить экспрессию гена ароматазы, которая стимулируется повышенным уровнем андрогенов. Отсутствие повышения экспрессии гена ароматазы было показано нами ранее в семенниках самцов крыс с моделью СД2 с ожирением, причем среди других генов тестикулярного стероидогенеза только экспрессия гена Star имела тенденцию к снижению, в то время как экспрессия генов, кодирующих цитохром CYP11A1 и дегидрогеназы HSD3b и HSD17b, не менялась, а экспрессия гена Cyp17a1 была повышена [21]. Необходимо отметить, что при потреблении самцами крыс Sprague Dawley диеты с высоким содержанием холестерина, приводящей к сильно выраженной гиперхолестеринемии, в семенниках снижалась экспрессия не только гена Star, как в нашем случае, но и генов обоих цитохромов, CYP11A1 и CYP17A1 [31, 32], что указывает на зависимость изменений в системе тестикулярного стероидогенеза от природы и степени нарушений липидного метаболизма, в том числе уровня холестерина, предшественника тестостерона и других стероидных гормонов.
Ослабление стероидогенной функции было ассоциировано с такими метаболическими и гормональными нарушениями, как повышение массы тела и жировой ткани, нарушенная толерантность к глюкозе, инсулиновая и лептиновая резистентность, дислипидемия, которые в настоящее время рассматриваются как основные патогенетические факторы репродуктивных расстройств при ожирении, метаболическом синдроме и СД2 с ожирением [2, 29, 33, 34]. Одним из подходов для улучшения метаболического статуса при СД2 и метаболическом синдроме является длительная терапия МФ. При этом имеются сведения о том, что МФ способен также нормализовать репродуктивные функции, хотя не все авторы это подтверждают [23–26]. Нами и другими авторами ранее было показано, что обработка МФ грызунов с различными формами диабета восстанавливает продукцию тестостерона и нормализует сперматогенез [21, 33–38]. В настоящем исследовании нами установлено, что, наряду с нормализацией ряда метаболических и гормональных показателей, МФ восстанавливает базовый уровень тестостерона у самцов крыс с ожирением, но при этом существенно не влияет на экспрессию стероидогенных генов и на стероидогенные эффекты ХГЧ и аллостерического ЛГР-агониста ТП03. В этой связи необходимо отметить, что, как ранее было показано, терапия МФ повышала стероидогенный эффект ХГЧ и ТП03 при их однократном введении самцам крыс с СД2, вызванным высокожировой диетой и низкой дозой стрептозотоцина, у которых выраженность метаболических расстройств превосходила таковую у крыс с ожирением [21]. Можно полагать, что эффективность МФ терапии в отношении чувствительности системы тестикулярного стероидогенеза к стимулирующему действию ЛГР-агонистов зависит от тяжести метаболического расстройства и выявляется в условиях более выраженных метаболических и гормональных нарушений [24]. Большое значение может играть и стратегия применения МФ, включая дозу препарата и продолжительность терапии [24, 38].
Интересен тот факт, что уровень экспрессии гена рецептора гонадолиберина в гипофизе группы “ОЖМ” значимо снижался в сравнении с группой “ОЖ”, что может быть обусловлено повышением продукции гонадолиберина при обработке крыс МФ и компенсаторным снижением рецептора компонента гонадолиберин-компетентной системы в гонадотрофах. Так, известно, что МФ, подобно лептину, активирует проопиомеланокортин-экспрессирующие нейроны, вовлеченные в позитивную регуляцию синтеза и секреции гонадолиберина, и, напротив, ингибирует нейроны, экспрессирующие нейропептид Y и агути-подобный пептид, которые негативно влияют на высвобождение гонадолиберина [39–42]. Обработка ХГЧ существенно подавляла экспрессию генов, кодирующих рецептор гонадолиберина и β-субъединицы ЛГ и ФСГ в гонадотрофах, что обусловлено запуском отрицательных обратных связей, в том числе вследствие повышения уровня тестостерона в крови. В то же время ТП03, для которого был характерен сравнительно мягкий стероидогенный эффект, в меньшей степени влиял на экспрессию генов LHbeta и FSHbeta, хотя и значимо снижал экспрессию гена рецептора гонадолиберина. Сравнительно давно ряд авторов, исследуя самцов крыс различного возраста, показали, что их обработка с помощью ХГЧ приводила к снижению экспрессии гена LHbeta в гипофизе и к значительному снижению уровня эндогенного ЛГ в крови, причем этот эффект зависел от дозы гонадотропина и степени повышения продукции андрогенов [43, 44].
В настоящем исследовании в семенниках всех изученных групп крыс с обработкой ХГЧ нами показано значительное подавление экспрессии гена, кодирующего ЛГР, что может быть обусловлено запуском даун-регуляции ЛГР при их длительной стимуляции экзогенными гонадотропинами с ЛГ-активностью [21, 44]. При этом ТП03, как и другие ранее изученные нами аллостерические ЛГР-агонисты, слабо влияет на экспрессию ЛГР [20, 21]. Это может быть обусловлено их более высокой селективностью в отношении внутриклеточных сигнальных каскадов, поскольку мишенями ТП03 и его аналогов являются в основном цАМФ-зависимые сигнальные пути (ЛГР/Gs-белок/аденилатциклаза/протеинкиназа А) [45, 46], в то время как другие пути, включая β-аррестиновые, ответственные за эндоцитоз и деградацию лиганд-активированных ЛГР, активируются аллостерическими ЛГР-агонистами в существенно меньшей степени [46, 47].
Таким образом, впервые показано, что ТП03, аллостерический агонист ЛГР на основе тиено[2,3-d]-пиримидина, демонстрирует умеренно выраженный стимулирующий эффект на тестикулярный стероидогенез у самцов крыс с ДИО, не оказывая ингибирующего влияния на экспрессию гена ЛГР в семенниках и умеренно снижая экспрессию гена ЛГ в аденогипофизе. Если при однократном введении ТП03 ДИО-крысам его стероидогенный эффект сопоставим с таковым в контрольной группе, то при длительном (5 дней) введении он существенно ему уступает. Стероидогенный эффект ХГЧ при его однократном введении крысам с ожирением значимо ниже, чем таковой в контроле, но сопоставим с ним при длительном введении гонадотропина, и существенно превосходит стероидогенные эффекты ТП03. В отличие от ТП03, ХГЧ значительно снижает экспрессию ЛГР в семенниках и в большей степени ингибирует экспрессию гена ЛГ в гонадотрофах, что указывает на отчетливо выраженную активацию отрицательных обратных связей в гипоталамо-гипофизарно-гонадной оси при обработке животных ХГЧ. Длительная МФ терапия частично восстанавливает андрогенный статус, при этом существенно не влияя на экспрессию генов стероидогенеза в семенниках. В условиях МФ терапии стероидогенные эффекты обоих изученных ЛГР-агонистов не повышаются, что указывает на отсутствие потенцирующего влияния на них длительной МФ обработки. Полученные результаты указывают на перспективы применения аллостерических и ортостерических ЛГР-агонистов для стимуляции тестикулярного стероидогенеза и на эффективность длительной МФ терапии для нормализации продукции тестостерона при ДИО, что может быть транслировано в клинику для коррекции репродуктивных расстройств, ассоциированных с ожирением и метаболическим синдромом. При этом совместное применение МФ и ЛГР-агонистов при ДИО представляется нецелесообразным, в отличие от СД2 с ожирением и характерными для него более тяжелыми метаболическими и гормональными нарушениями.
Список литературы
Dandona P, Dhindsa S, Chaudhuri A, Bhatia V, Topiwala S, Mohanty P (2008) Hypogonadotrophic hypogonadism in type 2 diabetes, obesity and the metabolic syndrome. Curr Mol Med 8: 816–828. https://doi.org/10.2174/156652408786733658
Fernandez CJ, Chacko EC, Pappachan JM (2019) Male Obesity-related Secondary Hypogonadism – Pathophysiology, Clinical Implications and Management. Eur Endocrinol 15: 83–90. https://doi.org/10.17925/EE.2019.15.2.83
Hermoso DAM, Bizerra PFV, Constantin RP, Ishii-Iwamoto EL, Gilglioni EH (2020) Association between metabolic syndrome, hepatic steatosis, and testosterone deficiency: evidences from studies with men and rodents. Aging Male 23: 1296–1315. https://doi.org/10.1080/13685538.2020.1764927
Louters M, Pearlman M, Solsrud E, Pearlman A (2022) Functional hypogonadism among patients with obesity, diabetes, and metabolic syndrome. Int J Impot Res 34: 714–720. https://doi.org/10.1038/s41443-021-00496-7
Van Cauwenberghe J, De Block C, Vanderschueren D, Antonio L (2023) Effects of treatment for diabetes mellitus on testosterone concentrations: A systematic review. Andrology 11: 225–233. https://doi.org/10.1111/andr.13318
Wagner IV, Oliver E, Dötsch J, Söder O (2020) Adverse effects of metabolic disorders in childhood on adult reproductive function and fertility in the male. J Pediatr Endocrinol Metab 34: 13–23. https://doi.org/10.1515/jpem-2020-0276
Gualtieri P, Tarsitano MG, DE Santis GL, Romano L, Esposito E, DE Lorenzo A (2022) Obesity in childhood: how to improve male adolescence incoming. Minerva Endocrinol (Torino) 47: 358–370. https://doi.org/10.23736/S2724-6507.21.03224-7
Baik M, Jeong JY, Park SJ, Yoo SP, Lee JO, Lee JS, Haque MN, Lee HJ (2020) Testosterone deficiency caused by castration increases adiposity in male rats in a tissue-specific and diet-dependent manner. Genes Nutr 15: 14. https://doi.org/10.1186/s12263-020-00673-1
Zhang Y, Qi J, Zhao J, Li M, Zhang Y, Hu H, Wei L, Zhou K, Qin H, Qu P, Cao W, Liu E (2023) Effect of Dietetic Obesity on Testicular Transcriptome in Cynomolgus Monkeys. Genes (Basel) 14: 557. https://doi.org/10.3390/genes14030557
Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Garcia-Galiano D, Castaño JP, Luque RM, Romero-Ruiz A, Castellano JM, Diéguez C, Pinilla L, Tena-Sempere M (2014) Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology 155: 1067–1079. https://doi.org/10.1210/en.2013-1584
Fink J, Matsumoto M, Tamura Y (2018) Potential application of testosterone replacement therapy as treatment for obesity and type 2 diabetes in men. Steroids 38: 161–166. https://doi.org/10.1016/j.steroids.2018.08.002
Giagulli VA, Castellana M, Carbone MD, Pelusi C, Ramunni MI, De Pergola G, Guastamacchia E, Triggiani V (2020) Weight loss more than glycemic control may improve testosterone in obese type 2 diabetes mellitus men with hypogonadism. Andrology 8: 654–662. https://doi.org/10.1111/andr.12754
Mongioì LM, Cimino L, Condorelli RA, Magagnini MC, Barbagallo F, Cannarella R, La Vignera S, Calogero AE (2020) Effectiveness of a Very Low-Calorie Ketogenic Diet on Testicular Function in Overweight/Obese Men. Nutrients 12: 2967 https://doi.org/10.3390/nu12102967
Caliber M, Saad F (2021) Testosterone therapy for prevention and reversal of type 2 diabetes in men with low testosterone. Curr Opin Pharmacol 58: 83–89. https://doi.org/10.1016/j.coph.2021.04.002
Jedamzik J, Bichler C, Felsenreich DM, Brugger J, Eichelter J, Nixdorf L, Krebs M, Itariu B, Langer FB, Prager G (2022) The male patient with obesity undergoing metabolic and bariatric surgery: changes in testosterone levels correlate with weight loss after one-anastomosis gastric bypass and Roux-en-Y gastric bypass. Surg Obes Relat Dis 30: S1550–7289(22)00830–00839. https://doi.org/10.1016/j.soard.2022.12.034
Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM (2010) Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 95: 2560–2575. https://doi.org/10.1210/jc.2009-2575
Ohlander SJ, Varghese B, Pastuszak AW (2018) Erythrocytosis Following Testosterone Therapy. Sex Med Rev 6: 77–85. https://doi.org/10.1016/j.sxmr.2017.04.001
Bond P, Smit DL, de Ronde W (2022) Anabolic-androgenic steroids: How do they work and what are the risks? Front Endocrinol (Lausanne) 13: 1059473. https://doi.org/10.3389/fendo.2022.1059473
Derkach KV, Dar’in DV, Lobanov PS, Shpakov AO (2014) Intratesticular, intraperitoneal, and oral administration of thienopyrimidine derivatives increases the testosterone level in male rats. Dokl Biol Sci 459: 326–329. https://doi.org/10.1134/S0012496614060040
Bakhtyukov AA, Derkach KV, Gureev MA, Dar’in DV, Sorokoumov VN, Romanova IV, Morina IY, Stepochkina AM, Shpakov AO (2020) Comparative study of the steroidogenic effect of human chorionic gonadotropin and thieno[2,3-d]-pyrimidine-based allosteric agonist of luteinizing hormone receptor in young adult, aging and diabetic male rats. Int J Mol Sci 21: 7493. https://doi.org/10.3390/ijms21207493
Bakhtyukov AA, Derkach KV, Sorokoumov VN, Stepochkina AM, Romanova IV, Morina IY, Zakharova IO, Bayunova LV, Shpakov AO (2021) The effects of separate and combined treatment of male rats with type 2 diabetes with metformin and orthosteric and allosteric agonists of luteinizing hormone receptor on steroidogenesis and spermatogenesis. Int J Mol Sci 23: 198. https://doi.org/10.3390/ijms23010198
Shpakov AO (2023) Allosteric Regulation of G-Protein-Coupled Receptors: From Diversity of Molecular Mechanisms to Multiple Allosteric Sites and Their Ligands. Int J Mol Sci 24: 6187. https://doi.org/10.3390/ijms24076187
Faure M, Bertoldo MJ, Khoueiry R, Bongrani A, Brion F, Giulivi C, Dupont J, Froment P (2018) Metformin in Reproductive Biology. Front Endocrinol (Lausanne) 9: 675. https://doi.org/10.3389/fendo.2018.00675
Shpakov AO (2021) Improvement Effect of Metformin on Female and Male Reproduction in Endocrine Pathologies and Its Mechanisms. Pharmaceuticals (Basel) 14: 42. https://doi.org/10.3390/ph14010042
Fernández-García JC, Barrios-Rodríguez R, Asenjo-Plaza M, Ramos-Molina B, Molina-Vega M, Guzmán-Guzmán A, Moreno-León L, Yubero-Serrano EM, Rius-Díaz F, Valdés S, Martínez-González MÁ, Jiménez-Moleón JJ, Tinahones FJ (2022) Metformin, testosterone, or both in men with obesity and low testosterone: A double-blind, parallel-group, randomized controlled trial. Metabolism 136: 155290. https://doi.org/10.1016/j.metabol.2022.155290
Tseng CH (2022) The Effect of Metformin on Male Reproductive Function and Prostate: An Updated Review. World J Mens Health 40: 11–29. https://doi.org/10.5534/wjmh.210001
Romanova IV, Derkach KV, Mikhrina AL, Sukhov IB, Mikhailova EV, Shpakov AO (2018) The leptin, dopamine and serotonin receptors in hypothalamic POMC-neurons of normal and obese rodents. Neurochem Res 43: 821–837. https://doi.org/10.1007/s11064-018-2485-z
Song MJ, Choi JY (2022) Androgen dysfunction in non-alcoholic fatty liver disease: Role of sex hormone binding globulin. Front Endocrinol (Lausanne) 13: 1053709. https://doi.org/10.3389/fendo.2022.1053709
Hsia SM, Chiang YF, Chen HY, Ali M, Wang PS, Wang KL (2022) Effect of High-Fructose Diet-Induced Metabolic Syndrome on the Pituitary-Gonadal Axis in Male Rats. Biomedicines 10: 3009. https://doi.org/10.3390/biomedicines10123009
El-Shehawi AM, El-Shazly S, Ahmed M, Alkafafy M, Sayed S, Farouk S, Alotaibi SS, Elseehy MM (2020) Transcriptome Analysis of Testis from HFD-Induced Obese Rats (Rattus norvigicus) Indicated Predisposition for Male Infertility. Int J Mol Sci 21: 6493. https://doi.org/10.3390/ijms21186493
Yu C, Jiang F, Zhang M, Luo D, Shao S, Zhao J, Gao L, Zuo C, Guan Q (2019) HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J Cell Mol Med 23: 3140–3150. https://doi.org/10.1111/jcmm.14143
Suleiman JB, Nna VU, Othman ZA, Zakaria Z, Bakar ABA, Mohamed M (2020) Orlistat attenuates obesity-induced decline in steroidogenesis and spermatogenesis by up-regulating steroidogenic genes. Andrology 8: 1471–1485. https://doi.org/10.1111/andr.12824
Barbagallo F, Condorelli RA, Mongioì LM, Cannarella R, Cimino L, Magagnini MC, Crafa A, La Vignera S, Calogero AE (2021) Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 11: 840. https://doi.org/10.3390/metabo11120840
Hasan H, Bhushan S, Fijak M, Meinhardt A (2022) Mechanism of Inflammatory Associated Impairment of Sperm Function, Spermatogenesis and Steroidogenesis. Front Endocrinol (Lausanne) 13: 897029. https://doi.org/10.3389/fendo.2022.897029
Nna VU, Bakar ABA, Ahmad A, Mohamed M (2019) Down-regulation of steroidogenesis-related genes and its accompanying fertility decline in streptozotocin-induced diabetic male rats: ameliorative effect of metformin. Andrology 7: 110–123. https://doi.org/10.1111/andr.12567
Derkach KV, Bakhtyukov AA, Romanova IV, Zorina II, Bayunova LV, Bondareva VM, Yu Morina I, Kumar Roy V, Shpakov AO (2020) The effect of metformin treatment on the basal and gonadotropin-stimulated steroidogenesis in male rats with type 2 diabetes mellitus. Andrologia 52: e13816. https://doi.org/10.1111/and.13816
Derkach KV, Bakhtyukov AA, Morina IY, Romanova IV, Bayunova LV, Shpakov AO (2022) Comparative Study of the Restoring Effect of Metformin, Gonadotropin, and Allosteric Agonist of Luteinizing Hormone Receptor on Spermatogenesis in Male Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus. Bull Exp Biol Med 172: 435–440. https://doi.org/10.1007/s10517-022-05409-2
Naghibi M, Tayefi Nasrabadi H, Soleimani Rad J, Gholami Farashah MS, Mohammadnejad D (2022) The effects of metformin and forskolin on sperm quality parameters and sexual hormones in type II diabetic male rats. Andrologia 54: 1605–1617. https://doi.org/10.1111/and.14426
Chau-Van C, Gamba M, Salvi R, Gaillard RC, Pralong FP (2007) Metformin inhibits adenosine 5'-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology 148: 507–511. https://doi.org/10.1210/en.2006-1237
Lee CK, Choi YJ, Park SY, Kim JY, Won KC, Kim YW (2012) Intracerebroventricular injection of metformin induces anorexia in rats. Diabetes Metab J 36: 293–299. https://doi.org/10.4093/dmj.2012.36.4.293
Lv WS, Wen JP, Li L, Sun RX, Wang J, Xian YX, Cao CX, Wang YL, Gao YY (2012) The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res 1444: 11–19. https://doi.org/10.1016/j.brainres.2012.01.028
Derkach K, Zakharova I, Zorina I, Bakhtyukov A, Romanova I, Bayunova L, Shpakov A (2019) The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS One 14: e0213779. https://doi.org/10.1371/journal.pone.0213779
Huhtaniemi IT, Warren DW, Catt KJ (1985) Regulation of infant and developing rat testicular gonadotropin and prolactin receptors and steroidogenesis by treatments with human chorionic gonadotropin, gonadotropin-releasing hormone analogs, bromocriptine, prolactin, and estrogen. Biol Reprod 32: 721–732. https://doi.org/10.1095/biolreprod32.4.721
Pakarinen P, Niemimaa T, Huhtaniemi IT, Warren DW (1994) Transcriptional and translational regulation of LH, prolactin and their testicular receptors by hCG and bromocriptine treatments in adult and neonatal rats. Mol Cell Endocrinol 101: 37–47. https://doi.org/10.1016/0303-7207(94)90217-8
Shpakov AO, Dar’in DV, Derkach KV, Lobanov PS (2014) The stimulating influence of thienopyrimidine compounds on the adenylyl cyclase systems in the rat testes. Dokl Biochem Biophys 456: 104–107. https://doi.org/10.1134/S1607672914030065
Derkach KV, Legkodukh AS, Dar’in DV, Shpakov AO (2017) The stimulating effect of thienopyrimidines structurally similar to Org 43553 on adenylate cyclase activity in the testes and on testosterone production in male rats. Cell Tissue Biol 11: 73–80. https://doi.org/10.1134/S1990519X17010035
van Koppen CJ, Zaman GJ, Timmers CM, Kelder J, Mosselman S, van de Lagemaat R, Smit MJ, Hanssen RG (2008) A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch Pharmacol 378: 503–514. https://doi.org/10.1007/s00210-008-0318-3
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова


