Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 11, стр. 1699-1717
Поведенческие, геномные и нейрохимические нарушения в модели нейротравмы на взрослых рыбах зебраданио (Danio rerio)
Н. П. Ильин 1, 2, 3, Д. С. Галстян 1, 2, 3, К. А. Демин 1, 2, А. В. Калуев 1, 2, 3, 4, 5, *
1 Институт трансляционной биомедицины, Санкт-Петербургский государственный университет,
Санкт-Петербург, Россия
2 Национальный медицинский исследовательский центр им. В.А. Алмазова МЗ РФ
Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий
им. акад. А.М. Гранова МЗ РФ
Санкт-Петербург, Россия
4 Направление “Нейробиология”, Научный центр генетики и наук о жизни,
Научно-технологический университет “Сириус”
Федеральная территория Сириус, Россия
5 Уральский федеральный университет
Екатеринбург, Россия
* E-mail: avkalueff@gmail.com
Поступила в редакцию 31.08.2023
После доработки 01.10.2023
Принята к публикации 02.10.2023
- EDN: HNGJDI
- DOI: 10.31857/S0869813923110043
Аннотация
Травматическое повреждение мозга (ТПМ, нейротравма) представляет собой серьезную биомедицинскую проблему, особенно в связи с высокой распространенностью и риском смертности. Поэтому необходимо понимание механизмов патогенеза ТПМ как в клинике, так в экспериментальных моделях на животных. В исследовании использовали модель проникающей травмы мозга (теленцефалона) для изучения поведенческих и молекулярных последствий ТПМ у взрослых рыб зебраданио (zebrafish, Danio rerio). Спустя четыре дня после индукции нейротравмы зебраданио демонстрировали гиполокомоцию в тесте незнакомого аквариума, нарушение рабочей памяти в Y-образном лабиринте и активацию экспрессии в теленцефалоне гена isg15, который является биомаркером повреждения нейронов. Кроме того, повреждение теленцефалона вызвало значительное снижение уровня норадреналина (но не дофамина и серотонина) в мозге зебраданио, что может отчасти объяснить наблюдаемые когнитивные дефициты, и подчеркивает потенциальное вовлечение нейротрансмиттерных систем в патогенез ТПМ.
ВВЕДЕНИЕ
Травматическое повреждение мозга (ТПМ) является повреждением ткани мозга в результате механического воздействия, варьируя от легких сотрясений до тяжелых, угрожающих жизни черепно-мозговых травм [1]. Ежегодно более 60 миллионов человек подвергаются ТПМ, и более 2% населения живут с вызванными им неврологическими нарушениями, позволяя говорить о своеобразной “тихой эпидемии” ТМП [2]. Данная гетерогенная группа патологических состояний имеет разнообразную этиологию и функциональные проявления [3]. Открытые ТПМ характеризуются видимыми повреждениями, кровотечением, возможной инфекцией и серьезными последствиями. Закрытая травма, в свою очередь, происходит без нарушения целостности черепа, но сам мозг при этом повреждается за счет деформации, сдавливания и растяжения [3]. Кроме того, ТПМ может быть классифицировано как очаговое или диффузное, в зависимости от распределения повреждения в мозге [4]. ТПМ влечет за собой ряд функциональных последствий, которые выходят за рамки острого периода травмы и могут сохраняться в течение длительного времени. В частности, распространенным эффектом ТПМ являются когнитивные дисфункции, в том числе нарушение внимания, памяти, исполнительных функций и скорости обработки информации [5]. Эти дисфункции являются характерным маркером ТПМ и наблюдаются как клинически, так и в разнообразных моделях на животных. При ТПМ также распространены моторные нарушения (затруднения двигательной активности и координации движений) [6], изменения эмоциональных состояний (перепады настроения, раздражительность, депрессия, тревога и эмоциональная неустойчивость) [7, 8], а также развитие целого ряда других заболеваний ЦНС, в том числе эпилепсии и болезней Альцгеймера и Паркинсона [9–11].
Эксайтотоксичность является одним из ключевых механизмов вторичного повреждения нейронов при ТПМ [12] и опосредована гиперактивацией рецепторов возбуждающих нейротрансмиттеров (прежде всего глутамата), повышающих внутриклеточную концентрацию кальция через ионотропные рецепторы [13]. Исследования на грызунах [14, 15] и на людях [16, 17] указывают на увеличение внеклеточной концентрации глутамата в мозге после ТПМ. Вероятно, при ТПМ происходит растяжение мембран нейронов, что создает микропоры, обеспечивающие приток ионов натрия внутрь клетки [12], это приводит к деполяризации мембраны, активации потенциал-зависимых кальциевых каналов и высвобождению глутамата во внеклеточное пространство, и, в итоге, избыточному притоку кальция в постсинаптические нейроны [18].
Увеличение внутриклеточной концентрации кальция нарушает нормальное функционирование клетки и активирует ее программируемую смерть [19]. Например, рост его концентрации приводит к активации кальций-зависимых протеаз, липаз и эндонуклеаз, которые инициируют разрушение клеточных структур [20], что приводит к нарушению синаптической передачи, дисфункции нейронных сетей, нарушению нейропластичности и расстройствам поведения, памяти и внимания.
Воспалительный процесс инициируется в первые минуты ТПМ в результате механического повреждения ткани, а также высвобождения молекулярных фрагментов, ассоциированных с повреждениями (DAMPs) [21, 22]. Первичное повреждение вызывает активацию резидентных иммунных клеток микроглии, которые служат первой линией защиты ЦНС. Микроглия быстро претерпевает морфологические и функциональные изменения, переходя из состояния покоя в активированное [23], высвобождая провоспалительные медиаторы (цитокины, хемокины и простагландины) и способствуя окислительному стрессу и нейродегенерации [24].
Астроциты также быстро реагируют на ТПМ, претерпевая морфологические и функциональные изменения (реактивный астроглиоз) [25] и образуя глиальный рубец, который действует как физический барьер, ограничивающий распространение повреждения. Активированные астроциты способны выделять провоспалительные медиаторы, а также наблюдается нарушение обратного захвата ими глутамата из внеклеточного пространства, что способствует эксайтотоксичности [26]. Нарушение целостности гематоэнцефалического барьера при ТПМ открывает возможность для инфильтрации в мозг периферических иммунных клеток – нейтрофилов, макрофагов и лимфоцитов, которые модулируют нейровоспаление, секретируя провоспалительные медиаторы [27]. В этом процессе особую роль играют фактор некроза опухоли-альфа (ФНО-α), интерлейкин-1β (ИЛ-β) и интерлейкин-6 (ИЛ-6), содержание которых в мозге стремительно растет в первые часы после ТПМ [28–31]. Эти цитокины способствуют выработке и высвобождению других провоспалительных медиаторов, усиливая нейровоспаление и нейроапоптоз.
Изучение патофизиологических аспектов ТПМ и их молекулярных механизмов требует широкого спектра экспериментальных моделей на животных. Помимо грызунов, следует отметить растущую роль альтернативных моделей ТПМ на рыбах зебраданио (zebrafish, Danio rerio) [32, 33]. Цель настоящей работы – валидировать на зебраданио модель ТПМ, вызванной проникающим повреждением теленцефалона, и оценить нейрохимические и геномные изменения в мозге зебраданио в данной модели.
МЕТОДЫ ИССЛЕДОВАНИЯ
Животные и их содержание
В исследовании использовали 36 взрослых зебраданио дикого коротко-плавникового типа, возраст 3–5 мес., которые были приобретены у компании ООО “Аксолотль” (Санкт-Петербург, Россия). Животные содержались в виварии Центра доклинических и трансляционных исследований Национального медицинского исследовательского центра им. В.А. Алмазова в соответствии со стандартными условиями [34]. Аутбредная линия дикого типа была использована в настоящем исследовании с учетом популяционной валидности модели патогенеза ЦНС. В частности, в то время как инбредные линии зебраданио более надежны для нейрогенетических исследований, моделирование патологий ЦНС предполагает достижение сходства с “реальными” заболеваниями человека, которые поражают генетически гетерогенные популяции. Таким образом, использование аутбредных зебраданио является более популяционно обоснованным и трансляционно релевантным подходом, соответствующим задачам настоящего исследования.
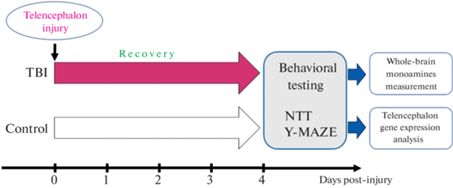
Для обеспечения оптимальных условий содержания использовалась автоматическая система распределения и очистки воды ZebTec Active Blue Stand (Tecniplast, West Chester, США). Рыбы размещались в ней группами по 15 особей в аквариумах объемом 3 литра. Температура воды автоматически поддерживалась на уровне 27 ± 0.5°C, а значение pH составляло 7.4. Интенсивность освещения составляла 950–960 люкс, с циклом свет/темнота 12/12 ч. Перед началом эксперимента животные прошли акклиматизацию в течение 2 недель. Все животные были экспериментально наивны и не подвергались никаким экспериментальным манипуляциям до начала данного исследования. Все рыбы принадлежали одной популяции и были случайным образом разделены на экспериментальные группы с помощью онлайн-генератора случайных чисел. Распределение рыб по группам, проведение поведенческих тестов и анализ данных выполнялись разными исследователями. При этом каждой группе были присвоены кодовые обозначения, что обеспечивало независимый анализ данных экспериментаторами относительно воздействия. Графическая иллюстрация плана экспериментов представлена на рис. 1.
Постановка модели проникающего повреждения теленцефалона
Постановка модели проводилась согласно методике, описанной ранее [35]. В начале эксперимента животные подвергались анестезии путем погружения в раствор трикаина (170 мг/мл этил-3-аминобензоат метансульфоната, Sigma Aldrich, St. Louis, МО, США) на 40–60 с, затем фиксировались с помощью губки, также смоченной в трикаине, и помещались под микроскоп. Травмирующее повреждение наносилось путем введения медицинской иглы (30 G) в медиальную область правой полусферы теленцефалона через крышу черепа на глубину 1.5–2 мм. После этого рыб выпускали в аквариум с чистой водой для восстановления. В результате описанных процедур выживаемость животных составляла 100%. Рыбы контрольной группы были экспериментально наивны, не подвергаясь экспериментальным воздействиям ранее (рис. 1).
Поведенческие тесты
Тест незнакомого аквариума был выбран как наиболее чувствительный и широко используемый метод для оценки параметров поведения и локомоции зебраданио [36, 37]. Он представлял собой акриловый прямоугольный аквариум размером 20 см (высота) × 20 см (длина) × 5 см (ширина), заполненный водой до уровня 19 см. Задняя и боковые стороны аквариума были окрашены в белый цвет для увеличения контрастности, а передняя сторона оставалась прозрачной. На расстоянии 1 м от аквариума располагалась видеокамера SJCAM SJ4000 (SJCAM Ltd., Шэньчжэнь, Китай). Запись производилась в течение 5 мин (после помещения зебраданио в аквариум) с частотой 60 кадров в секунду. Далее с помощью программного обеспечения Noldus IT EthoVision XT11.5 (Noldus IT, Wageningen, Нидерланды) были проанализированы поведенческие параметры зебраданио из сделанных видеозаписей: преодоленная дистанция (см), средняя скорость (см/с), время, проведенное в неподвижном состоянии (с), латентный период первого заплыва в верхнюю часть аквариума (с), время, проведенное в верхней половине аквариума (с) и количество переходов в верхнюю половину аквариума.
Y-образный лабиринт является распространенным тестом для оценки рабочей памяти зебраданио [38]. В ходе тестирования рыб помещали по одной в белые акриловые Y-образные аквариумы, заполненные водой. Каждый аквариум состоял из трех идентичных рукавов (длина 50, ширина 20, высота 140 мм), расположенных под углом 120° друг к другу. Рыбы имели возможность свободно исследовать все ответвления лабиринта в течение 15 мин, при этом их перемещение фиксировалось на камеру. За это время животное делало определенное количество дискретных выборов (как правило, более 100) – поворотов налево (L) или направо (R). Полученные видеозаписи анализировались в программе Noldus Ethovision, фиксируя время, когда рыба заплывала в каждое плечо (рукав) лабиринта и когда покидала его. Эти данные были преобразованы в последовательность символов L и R, обозначающих левые и правые повороты соответственно [39]. Полученные для каждой рыбы последовательности были далее преобразованы в распределение по 16 тетрад (LLLL, LLLR, LLRL, LLRR, LRLL, LRLR, LRRL, LRRR, RLLL, RLLR, RLRL, RLRR, RRLL, RRLR, RRRL, RRRR), нормированным к общему количеству оборотов и выраженным в %, согласно стандартным методикам [38].
Полимеразная цепная реакция в реальном времени (ПЦР-РВ)
Забор проб теленцефалона проводился непосредственно после проведения поведенческих экспериментов. Рыб умерщвляли путем погружения в ледяную воду, до полной остановки дыхательных движений, после чего под микроскопом вскрывали крышу черепа и извлекали конечный мозг. Выделение РНК осуществлялось с использованием реагента ExtractRNA (Евроген, Москва, Россия) по стандартному протоколу для TriZol-опосредованной экстракции. Обратная транскрипция была выполнена с помощью коммерческого набора MMLV RT kit (Евроген). Для проведения ПЦР-РВ использовали коммерческую реакционную смесь 5X qPCRmix-HS SYBR (Евроген). Сама реакция проводилась в системе ПЦР реального времени CFX96 Touch (Bio-Rad laboratories, Hercules, CA, США). Нуклеотидные последовательности праймеров, используемых в данной работе, приведены в табл. 1.
Таблица 1.
Нуклеотидные последовательности праймеров, использованных в работе
| Целевой ген | Прямой праймер | Обратный праймер |
|---|---|---|
| eef1a1l1 | CCCAGTGCTGGATTGCCACA | GCGGCATCTCCGGATTTGAG |
| casp3a | CCAGGGTCAACCATAAAGTAGC | TCTTTGGTGAGCATTGAGACGA |
| Tnfa | GGGCAATCAACAAGATGGAAG | GCAGCTGATGTGCAAAGACAC |
| isg15 | ACTTGATTTCGGTGCGACTTGC | GCTGCATCGTCACCGAGTTAT |
Дизайн праймеров был осуществлен с использованием базы данных National Center for Biotechnology Information (NCBI) Primer-BLAST (Basic Local Alignment Search Tool) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Для расчета изменения относительной экспрессии генов использовался метод 2–∆∆Ct [40]. В качестве референсного гена был выбран eef1a1l1 – ген домашнего хозяйства, кодирующий эукариотический фактор инициации трансляции.
Высокоэффективная жидкостная хроматография
Высокоэффективная жидкостная хроматография с электрохимической детекцией (ВЭЖХ-ЭХД) использовалась для анализа содержания моноаминов (норадреналина, дофамина, серотонина) и их метаболитов (3,4-дигидроксифенилуксусной кислоты – ДОФУК, гомованилиновой кислоты – ГВК и 5-гидроксииндолуксусной кислоты – 5-ГИУК) в пробах головного мозга зебраданио. Методика основана на модифицированной версии протокола, разработанного [41]. Анализ проводился на хроматографе HTEC-500 (Eicom, San Diego, США) с использованием колонки EICOMPAK CA-5ODS. Подвижная фаза для ВЭЖХ-ЭХД состояла из 0.1 М фосфатного буфера (pH 6.0), содержащего октансульфонат натрия (1.9 мМ), этилендиаминтетрауксусую кислоту (0.17 мМ) и метанол (1.13 М), pH подвижной фазы – 4.5. Для нормализации данных был использован внутренний стандарт – 1%-ный раствор 3,4-дигидроксибензиламина в 0.1 М HClO4 (добавлялся в пробы в соотношении 10 мкл на 1 мг ткани) и внешний стандарт – 0.1 М растворы серотонина, дофамина, норадреналина, ДОФУК, ГВК, 5-ГИУК и 3,4-дигидроксибензиламина (10 мкл каждого раствора на 930 мкл 0.1 М HClO4). Все реактивы для ВЭЖХ были получены из Sigma Aldrich, St. Louis, США.
Статистическая обработка данных
Полученные данные анализировали с использованием языка программирования R (версия 4.2.1) в среде R-Studio (версия 2022.02.2). Проверка данных на нормальность проводилась с помощью теста Колмогорова–Смирнова. Ввиду отсутствия нормального распределения у исследуемых переменных, данные теста незнакомого аквариума, хроматографии и ПЦР анализировались с использованием непараметрического теста Вилкоксона–Манна–Уитни для независимых выборок. Для анализа распределения тетраграмм переходов в Y-образном лабиринте была построена обобщенная линейная модель (GLM, Poisson distribution, log link), где факторы “Группа”, “Тетраграмма”, и взаимодействие “Группа” × “Тетраграмма” использовались в качестве предикторов. При обнаружении значимого влияния одного из факторов или когда взаимодействие между факторами было значимым, проводилось попарное сравнение с использованием оцененных маргинальных средних (пакет R “emmeans”, коррекция значения p по методу Сидака). Различия считались статистически значимыми при значении p < 0.05.
Расчет размера выборки (n) проводился на основании данных пилотного исследования с использованием функции pwrss.np.2groups пакета pwrss в R. Данная функция позволяет определить примерное n для теста Вилкоксона с учетом желаемой мощности теста (0.80), уровня значимости (0.05), а также значений среднего и стандартного отклонения среднего выбранной переменной для двух групп. В качестве зависимой переменной была выбрана дистанция в тесте незнакомого аквариума как один из наиболее важных поведенческих показателей. В пилотном исследовании значения среднего и стандартного отклонения среднего составили 954 ± 371 см для контрольной группы и – 643 ± 245 см для ТПМ (размер эффекта – 0.478). На основании этих данных было получено значение n = 16, которое было скорректировано до n = 18 с учетом возможной смертности в результате экспериментальных воздействий. В ходе анализа данных удаления выбросов не проводилось. Ввиду повреждения видеофайлов результаты теста незнакомого аквариума не были получены для трех животных (два из контрольной группы и одно из группы ТПМ). Критерием для исключения из финального анализа в тесте Y-образного лабиринта являлось меньшее, чем 100 число поворотов за время теста (отмечалось для двух зебраданио из каждой группы).
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Влияние ТПМ на поведение в тесте незнакомого аквариума
ТПМ оказало статистически значимое влияние на поведение зебраданио в тесте незнакомого аквариума, что выражалось в изменении дистанции, числа переходов между зонами и времени нахождения в верхней половине аквариума (рис. 2 и табл. 2). Рыбы, подвергнутые ТПМ, преодолевали меньшую дистанцию за время теста, а также совершали меньшее число переходов между зонами в сравнении с контрольной группой (p < 0.05), что говорит о снижении локомоторной активности. В то же время травма мозга привела к увеличению времени, проведенному в верхней половине аквариума (p < 0.05).
Рис. 2.
Влияние травматического повреждения мозга (ТПМ, TBI) на поведение зебраданио в тесте незнакомого аквариума. Control – контрольная группа. Оценивали суммарное расстояние, преодоленное животным за время теста (Distance), число переходов между верхней и нижней половинами аквариума (Zone transitions) и суммарное время в верхней половине аквариума (Top duration). Данные представлены как медиана ± межквартильный размах (n = 16–17 в группе) и проанализированы тестом Вилкоксона-Манна-Уитни (Wilcoxon).
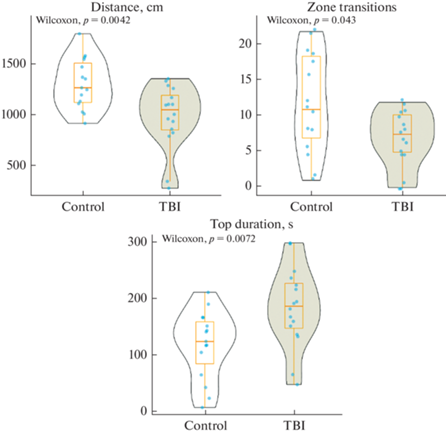
Таблица 2.
Сравнение ключевых поведенческих показателей теста незнакомого аквариума у зебраданио
| Группа | Медиана | Q1 | Q3 | IQR | Критерий Вилкоксона, W | Значение р |
|---|---|---|---|---|---|---|
| Дистанция (см) | ||||||
| Контроль | 1274.35 | 1130.12 | 1515.2 | 385.08 | 191 | <0.01 |
| ТПМ | 1059.64 | 860.83 | 1200.11 | 339.27 | ||
| Количество переходов между зонами | ||||||
| Контроль | 11 | 7 | 18.5 | 11.5 | 171.5 | <0.05 |
| ТПМ | 7.5 | 5 | 10.25 | 5.25 | ||
| Время в верхней половине аквариума (с) | ||||||
| Контроль | 126.4 | 87.2 | 161.13 | 73.93 | 51.5 | <0.01 |
| ТПМ | 188.6 | 149.9 | 228.9 | 79 | ||
Влияние ТПМ на поведение в Y-образном лабиринте.
Анализ обобщенной линейной модели показал значимое влияние фактора “тетраграмма” (p < 0.001), а также взаимодействия факторов “тетраграмма” × ”группа” (p < 0.001) на процентное распределение альтернативных выборов зебраданио в Y‑образном лабиринте (рис. 3). Так, рыбы контрольной группы демонстрировали естественное чередование (спонтанную альтернацию) левых и правых поворотов, о чем свидетельствует характерное распределение переходов с преобладанием тетраграмм LRLR и RLRL (рис. 2, табл. 3). Подобное распределение соответствует типичному поведению рыб в данном тесте, описанному в литературе [38, 42]. В группе ТПМ спонтанная альтернация не была выражена, о чем говорит значительно меньший процент тетрад LRLR и RLRL в сравнении с контрольной группой (p < 0.001). При этом рыбы из группы ТПМ показали большую склонность к повторению левых поворотов, о чем говорит увеличение процентного отношения тетраграмм LLLL в сравнении с контрольной группой (p < 0.001, рис. 3, табл. 3).
Рис. 3.
Влияние травматического повреждения мозга (ТПМ, TBI) на распределение тетраграмм альтернативных выборов в тесте Y-образного лабиринта (Tetragrams frequency distribution, n = 16 в группе). Control – контрольная группа. Данные представлены как среднее ± стандартная ошибка среднего. *** – p < 0.001 (попарное сравнение оцененных маргинальных средних значений с коррекцией значений p методом Сидака для множественных сравнений).
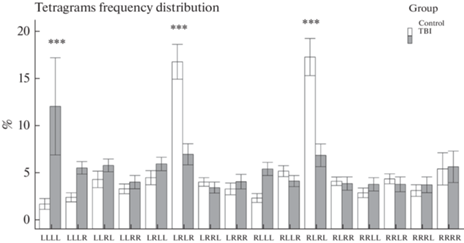
Таблица 3.
Сравнение частоты тетраграмм в тесте Y-образного лабиринта между экспериментальными группами зебраданио
| Сравнение | Тетраграммы | Estimate | SE | z-ratio | p value |
|---|---|---|---|---|---|
| Контроль–ТПМ | LLLL | –1.59 | 0.29 | –5.52 | <0.001 |
| Контроль–ТПМ | LLLR | –0.66 | 0.29 | –2.29 | 0.02 |
| Контроль–ТПМ | LLRL | –0.25 | 0.25 | –1 | 0.32 |
| Контроль–ТПМ | LLRR | –0.15 | 0.28 | –0.55 | 0.58 |
| Контроль–ТПМ | LRLL | –0.24 | 0.25 | –0.97 | 0.33 |
| Контроль–ТПМ | LRLR | 0.81 | 0.18 | 4.41 | <0.001 |
| Контроль–ТПМ | LRRL | 0.13 | 0.28 | 0.46 | 0.65 |
| Контроль–ТПМ | LRRR | –0.17 | 0.28 | –0.59 | 0.56 |
| Контроль–ТПМ | RLLL | –0.56 | 0.29 | –2.28 | 0.06 |
| Контроль–ТПМ | RLLR | 0.19 | 0.26 | 0.74 | 0.46 |
| Контроль–ТПМ | RLRL | 0.85 | 0.18 | 4.64 | <0.001 |
| Контроль–ТПМ | RLRR | 0.05 | 0.27 | 0.18 | 0.86 |
| Контроль–ТПМ | RRLL | –0.21 | 0.29 | –0.72 | 0.47 |
| Контроль–ТПМ | RRLR | 0.12 | 0.27 | 0.43 | 0.67 |
| Контроль–ТПМ | RRRL | –0.14 | 0.29 | –0.48 | 0.63 |
| Контроль–ТПМ | RRRR | –0.03 | 0.24 | –0.14 | 0.89 |
Статистически значимые различия выделены жирным шрифтом (попарное сравнение оцененных маргинальных средних значений с коррекцией значений p методом Сидака для множественных сравнений). Estimate – разница между оцененными маргинальными средними, SE – стандартная ошибка среднего, ТПМ – травматическое повреждение мозга.
Дальнейший анализ распределения тетраграмм выявил статистически значимые различия между группами по ключевым показателям теста – процентному отношению чередований (тетраграммы LRLR + RLRL) и повторений (тетраграммы LLLL + RRRR). Так, зебраданио из группы ТПМ показали увеличение процента повторений относительно контрольной группы и снижение процента чередований (p < 0.05, рис. 4 и табл. 4). Данные изменения принято интерпретировать как нарушение спонтанной альтернации как показателя рабочей памяти у зебраданио в данном тесте [38].
Рис. 4.
Влияние травматического повреждения мозга (ТПМ, TBI) на поведение зебраданио в тесте Y-образного лабиринта, в том числе процентное отношение чередований (Alternations) и повторений (Repetitions) левых и правых поворотов. Control – контрольная группа. Данные представлены как медиана ± межквартильный размах (n = 16 в группе) и проанализированы тестом Вилкоксона-Манна-Уитни (Wilcoxon).
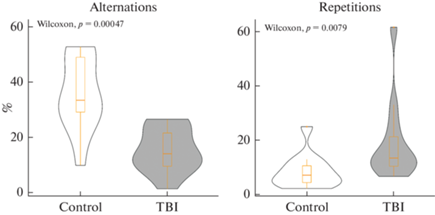
Таблица 4.
Сравнение ключевых поведенческих показателей спонтанной альтернации в тесте Y-образного лабиринта у зебраданио
| Группа | Медиана | Q1 | Q3 | IQR | Критерий Вилкоксона, W | Значение р |
|---|---|---|---|---|---|---|
| Повторения (%) | ||||||
| Контроль | 7.58 | 4.9 | 11.04 | 6.14 | 25.5 | <0.01 |
| ТПМ | 13.85 | 10.86 | 21.65 | 10.79 | ||
| Чередования (%) | ||||||
| Контроль | 33.7 | 29.42 | 49.28 | 19.86 | 133 | <0.001 |
| ТПМ | 14.36 | 9.84 | 21.87 | 12.03 | ||
Влияние ТПМ на содержание моноаминов и их метаболитов в мозге зебраданио
Анализ содержания моноаминов (норадреналина, дофамина и серотонина), а также их метаболитов (гомованилиновой кислоты, 5-гидроксииндолуксусной кислоты и диоксифенилуксусной кислоты) в целом мозге зебраданио представлены на рис. 5 и в табл. 5. Травматическое повреждение теленцефалона вызвало статистически значимое снижение уровня норадреналина в мозге зебраданио (p < 0.05 против контрольной группы), при этом содержание других анализируемых веществ между группами не различалось (p > 0.05).
Рис. 5.
Влияние травматического повреждения мозга (ТПМ, TBI) на содержание моноаминов и их метаболитов в целом мозге зебраданио (n = 10 в группе). Control – контрольная группа. Данные представлены как медиана ± межквартильный размах и проанализированы тестом Вилкоксона-Манна-Уитни (Wilcoxon). Norepinephrine – норадреналин, Dopamine – дофамин, Serotonin – серотонин, HVA – гомованилиновая кислота, DOPAC – дигидроксифенилуксусная кислота, 5-HIAA – 5-гидроксииндолуксусная кислота.
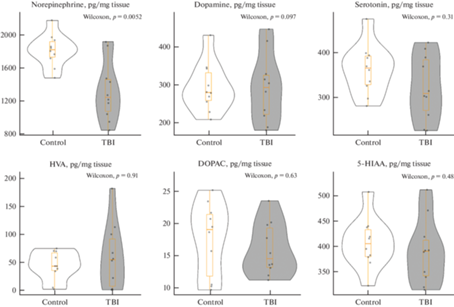
Таблица 5.
Сравнение содержания моноаминов в мозге зебраданио на фоне нейротравмы
| Группа | Медиана | Q1 | Q3 | IQR | Критерий Вилкоксона, W | Значение р |
|---|---|---|---|---|---|---|
| Норадреналин (пг/мг ткани) | ||||||
| Контроль | 1825.61 | 1736.71 | 1922.6 | 185.89 | 86 | 0.005 |
| ТПМ | 1250.67 | 1082.43 | 1466 | 383.56 | ||
| Дофамин (пг/мг ткани) | ||||||
| Контроль | 281.42 | 260.62 | 333.32 | 72.7 | 51 | 0.971 |
| ТПМ | 294.76 | 224.18 | 326.57 | 102.38 | ||
| Серотонин (пг/мг ткани) | ||||||
| Контроль | 367.21 | 330.52 | 394.06 | 63.54 | 64 | 0.315 |
| ТПМ | 310.89 | 273.36 | 385.85 | 112.49 | ||
| ГВК (пг/мг ткани) | ||||||
| Контроль | 44.11 | 36.23 | 67.7 | 31.47 | 48 | 0.912 |
| ТПМ | 55.5 | 8.36 | 92.27 | 83.91 | ||
| ДОФУК (пг/мг ткани) | ||||||
| Контроль | 19.07 | 11.85 | 21.41 | 9.56 | 57 | 0.631 |
| ТПМ | 14.57 | 13.22 | 19.29 | 06.08 | ||
| 5-ГИУК (пг/мг ткани) | ||||||
| Контроль | 405.9 | 380.46 | 433.45 | 52.99 | 60 | 0.481 |
| ТПМ | 391.03 | 342.72 | 412.89 | 70.17 | ||
Статистически значимые различия между группами выделены жирным шрифтом (критерий Вилкоксона–Манна–Уитни, p < 0.05, n = 10 в группе). Q1 – первый квартиль, Q3 – третий квартиль, IQR – межквартильный размах. ТПМ – травматическое повреждение мозга, ГВК – гомованилиновая кислота, ДОФУК – дигидроксифенилуксусная кислота, 5-ГИУК – 5-гидроксииндолуксусная кислота.
Влияние ТПМ на экспрессию генов casp3a, tnfa и isg15 в теленцефалоне зебраданио
Анализ результатов ПЦР-РВ показал, что травматическое повреждение оказало статистически значимое влияние на уровень экспрессии гена isg15 в теленцефалоне зебраданио (p < 0.001 против контрольной группы), при этом изменений в экспрессии генов casp3 и tnfa обнаружено не было (p > 0.05; рис. 6 и табл. 6).
Рис. 6.
Влияние травматического повреждения мозга (TBI) на относительный уровень экспрессии (fold change) генов casp3a, tnfa и isg15 в теленцефалоне зебраданио (n = 8 в группе). Данные нормированы относительно гена eef1a1l1. Control – контрольная группа. Данные представлены как медиана ± межквартильный размах.
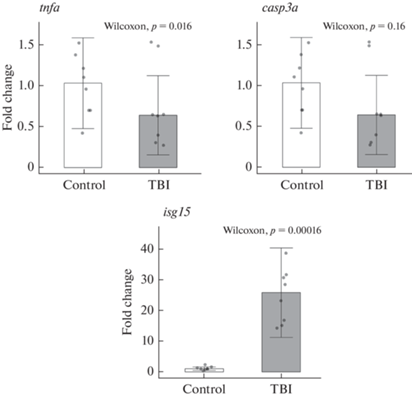
Таблица 6.
Сравнение экспрессии генов casp3a, tnfa и isg15 в теленцефалоне зебраданио на фоне нейротравмы
| Группа | Медиана | Q1 | Q3 | IQR | Критерий Вилкоксона, W | Значение р |
| casp3a (относительный уровень экспрессии) | ||||||
| Контроль | 1.04 | 0.7 | 1.26 | 0.56 | 46 | 0.16 |
| ТПМ | 0.64 | 0.38 | 0.86 | 0.49 | 46 | |
| tnfa (относительный уровень экспрессии) | ||||||
| Контроль | 0.54 | 0.45 | 01.01 | 0.56 | 42 | 0.33 |
| ТПМ | 0.4 | 0.26 | 0.79 | 0.52 | 42 | |
| isg15 (относительный уровень экспрессии) | ||||||
| Контроль | 1.03 | 0.8 | 1.39 | 0.59 | 0 | <0.001 |
| ТПМ | 25.81 | 16.37 | 30.93 | 14.55 | 0 | |
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Несмотря на высокую гетерогенность клинических форм ТПМ, можно выделить некоторые общие закономерности, присущие всем видам данной патологии [43]. Первичные ТПМ возникают при физическом воздействии и вызывают разрушение оболочек мозга и гематоэнцефалического барьера, разрыв кровеносных сосудов, растяжение и разрыв аксонов, гибель нейронов и глиальных клеток [44]. В ходе первичных повреждений происходит неспецифическая гибель клеток. Первичные повреждения инициируют возникновение вторичных повреждений, которые проявляются в системных нарушениях (отек мозга, увеличение внутричерепного давления, ишемия), а также активацией физиологических и клеточных механизмов, таких как эксайтотоксичность, нейровоспаление, окислительный стресс, митохондриальная дисфункция, программируемая клеточная смерть [12]. Вторичные повреждения могут развиваются в течение часов, дней или даже недель после первичной травмы, а также выходить за пределы места первоначального повреждения, затрагивая соседние и отдаленные области мозга, и именно от характера и тяжести вторичных повреждений во многом зависят итоговые функциональные последствия ТПМ.
Хотя ТПМ приводит к неспецифической смерти нервных клеток в результате некроза в первые минуты после травмы, клинические и экспериментальные данные указывают на важность “второй волны” клеточной гибели за счет апоптоза и других механизмов программируемой смерти клеток [45]. При ТПМ происходят характерные апоптотические изменения морфологии клеток (сморщивание, фрагментация ядер, конденсация хроматина, образование апоптотических телец), а также маркеров фрагментации ДНК и активации проапоптотических белков [46–50], прежде всего каспаз, которые расщепляют белки и вызывают гибель клеток (каспазы 3, 6 и 7) либо активируют другие каспазы (каспазы 2, 8 и 9) [51]. При ТПМ наиболее распространенной причиной апоптоза является чрезмерный уровень внутриклеточного кальция, вызванный эксайтотоксичностью [12], что приводит к активации каспаз и разрушениям клеточных структур при апоптозе. Кроме того, апоптоз инициируется связыванием лигандов смерти (ФНО-α) с соответствующими рецепторами [52], также активируя каскад каспаз и, в результате, апоптоз. Помимо апоптоза, описано еще несколько механизмов программируемой смерти клеток, активируемых в результате ТПМ, в том числе некроптоз, аутофагия, пироптоз и ферроптоз [53–55].
В настоящем исследовании травматическое повреждение конечного мозга зебраданио привело к выраженным поведенческим нарушениям, свидетельствуя об успешности постановки модели нейротравмы. Так, на 4-й день после ТПМ рыбы демонстрировали гиполокомоцию, выраженную в снижении преодоленной дистанции в тесте незнакомого аквариума, а также в нарушении рабочей памяти, выраженное в снижении относительного количества чередований и увеличении количества повторений в тесте Y-образного лабиринта. Эти данные согласуются с результатами других работ на модели зебраданио, в которых повреждение мозга вызывало подобные поведенческие изменения [56–58], а также с моделями ТПМ на млекопитающих, в которых когнитивные и моторные нарушения являются одними из наиболее распространенных функциональных последствий травмы мозга [59–61].
Интересным наблюдением является значительное увеличение экспрессии isg15 в группе с ТПМ (рис. 5). Данный ген кодирует убиквитин-подобный белок ISG15 (interferon-stimulated gene 15), который может связываться с другими белками-мишенями в процессе ISG-илирования (ISGylation), похожем на убиквитинирование [62]. Показано, что ISG15 может играть важную роль в патогенезе ТПМ, о чем свидетельствует многократное увеличение экспрессии его гена ISG15, а также степени ISG-илирования белков в моделях нейротравмы на мышах и пациентов с ТПМ [63]. Более того, активация этого гена наблюдается при повреждениях нервной ткани различной этиологии, что позволяет говорить о ISG15 как об общем маркере повреждений нейронов. Тем не менее, физиологическая функция ISG15, а также функциональные последствия его активации в ответ на травму, еще плохо изучены [64]. Результаты настоящей работы впервые указывают на активацию системы ISG15 при ТПМ у зебраданио, что свидетельствует о высокой эволюционной консервативности данного ответа, и позволяет использовать зебраданио в качестве модели для ISG15-ассоциированных патологий.
Любопытно, что повреждение теленцефалона не оказало влияния на уровень экспрессии генов каспазы-3 и ФНО-α (рис. 5). Каспаза-3 является ключевым эффектором апоптоза, а ФНО-α – важным провоспалительным цитокином, участвующим в инициации нейровоспаления при ТПМ, а также индуктором апоптоза. Увеличение экспрессии генов этих биомаркеров описано в моделях ТПМ как на рыбах [65–67], так и на млекопитающих [28, 68–71]. В настоящей работе отсутствие эффекта на них после примененного ТПМ может быть связано с более поздним сроком забора проб для анализа (4 дня после травмы), когда основная острая фаза воспалительного ответа уже миновала. В пользу такого предположения говорит резкое увеличение уровня экспрессии tnfa в первые часы после травмы, сменившееся постепенным снижением, с возвратом к физиологическим значениям на 3- 4-й день в той же модели ТПМ [66]. Увеличение экспрессии гена каспазы-3 в модели ТПМ рыб наблюдалось через 20 ч после повреждения, но не через 3 дня [65]. Аналогично, в моделях на грызунах увеличение экспрессии гена ФНО-α наблюдается в первые часы после травмы мозга, после чего этот показатель возвращается к норме [28, 68]. Напротив, увеличение экспрессии гена каспазы-3, а также содержания активированной формы этого белка у млекопитающих наблюдается через дни или даже месяцы с момента травмы [69–71]. Таким образом, настоящее исследование указывает как на сходства, так и на различия молекулярного ответа на нейротравму у зебраданио и млекопитающих.
Наконец, повреждение теленцефалона вызвало значительное снижение уровня норадреналина в ткани головного мозга зебраданио (рис. 4). У зебраданио, как и у млекопитающих, тела норадренергических нейронов локализованы в голубом пятне ствола головного мозга, откуда восходящие проекции направляются к другим отделам [72]. Учитывая локальность наносимой травмы, наблюдаемые нейрохимические изменения, вероятнее всего, связаны со вторичными повреждениями, вызванными ТПМ, нежели с механическим повреждением нервной ткани. Норадренергические нейроны, наряду с дофаминергическими, наиболее подвержены метаболическим нарушениям, возникающим в результате их повреждения [73]. Так, норадренергические нейроны голубого пятна демонстрируют высокую чувствительность к окислительному стрессу, вызванному нейровоспалением [74]. Показано снижение содержания норадреналина и уменьшение числа норадренергических нейронов в стволе мозга в ответ на проникающую травму сенсомоторной коры у крыс, что может быть связано с окислительным стрессом [75]. Поскольку норадреналин играет важную роль в регуляции многих функций ЦНС [76], снижение уровня этого моноамина в мозге зебраданио может быть отчасти ответственно за наблюдаемые в работе когнитивные и моторные нарушения.
В целом, перспективность использования зебраданио в моделях ТПМ связана с сочетанием этим организмом как эволюционно консервативных, так и отличных от млекопитающих особенностей. Так же, как у грызунов, ТПМ у зебраданио вызывает развитие вторичных повреждений, связанных с эксайтотоксичностью, нейровоспалением, окислительным стрессом и апоптозом [57, 77, 78]. При этом рыбы обладают несопоставимо большей способностью к нейрорегенерации за счет интенсивного нейрогенеза – мозг взрослой зебраданио содержит 16 зон активной пролиферации нейронов в сравнении с двумя у млекопитающих [79]. Таким образом, в то время как у млекопитающих ТПМ приводит к перманентному разрушению нейронных связей, аккумуляции вторичных повреждений и образованию глиального рубца, зебраданио способны достаточно быстро регенерировать обширные повреждения без долгосрочных последствий [35]. Раскрытие молекулярных механизмов регенерации мозга у рыб может позволить определить потенциальные терапевтические мишени, способствующие репарации нейронов и функциональному восстановлению при повреждениях ЦНС у человека. Кроме того, сравнительный анализ ответа на травму мозга у зебраданио и млекопитающих может дать ценную информацию о барьерах, препятствующих нейрорегенерации у высших позвоночных.
ЗАКЛЮЧЕНИЕ
В целом настоящее исследование показало, что нейротравма конечного мозга зебраданио вызывает когнитивные и моторные нарушения, увеличение экспрессии isg15 и снижение содержания норадреналина в мозге. Поведенческие изменения, наблюдаемые в настоящей модели, согласуются с таковыми в моделях на млекопитающих и у пациентов с ТПМ, что свидетельствует о ее трансляционном потенциале. Для зебраданио активация экспрессии isg15 в ответ на травму была показана впервые, что может указывать на наличие эволюционно консервативного механизма ответа на травму ЦНС, опосредованного ISG15. Кроме того, было впервые описано влияние ТПМ на состояние моноаминергических систем зебраданио, что является важным дополнением к существующему описанию нейрохимических изменений в данной модели при нейротравмах.
Список литературы
Risdall JE, Menon DK (2011) Traumatic brain injury. Philos Trans R Soc B Biol Sci 366: 241–250. https://doi.org/10.1098%2Frstb.2010.0230
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM (2018) Estimating the global incidence of traumatic brain injury. J Neurosurg 130: 1080–1097. https://doi.org/10.3171/2017.10.JNS17352
Parikh S, Koch M, Narayan RK (2007) Traumatic brain injury. Int Anesthesiol Clin 45: 119–135. https://doi.org/10.1097/AIA.0b013e318078cfe7
Andriessen TM, Jacobs B, Vos PE (2010) Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J Cell Mol Med 14: 2381–2392. https://doi.org/10.1111/j.1582-4934.2010.01164.x
Walker KR, Tesco G (2013) Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci 5: 29. https://doi.org/10.3389/fnagi.2013.00029
Galea OA, Cottrell MA, Treleaven JM, O’Leary SP (2018) Sensorimotor and physiological indicators of impairment in mild traumatic brain injury: a meta-analysis. Neurorehabil Neural Repair 32: 115–128. https://doi.org/10.1177/1545968318760728
Mallya S, Sutherland J, Pongracic S, Mainland B, Ornstein TJ (2015) The manifestation of anxiety disorders after traumatic brain injury: a review. J Neurotrauma 32: 411–421. https://doi.org/10.1089/neu.2014.3504
Yang C-C, Hua M-S, Lin W-C, Tsai Y-H, Huang S-J (2012) Irritability following traumatic brain injury: divergent manifestations of annoyance and verbal aggression. Brain Injury 26: 1185–1191. https://doi.org/10.3109/02699052.2012.666374
Delic V, Beck KD, Pang KC, Citron BA (2020) Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol Commun 8: 1–16. https://doi.org/10.1186/s40478-020-00924-7
Ding K, Gupta PK, Diaz-Arrastia R (2016) Epilepsy after traumatic brain injury. Transl Res Trauma Brain Injury Chapter 14. https://doi.org/10.1201/b18959-19
Sivanandam TM, Thakur MK (2012) Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev 36: 1376–1381. https://doi.org/10.1016/j.neubiorev.2012.02.013
Krishnamurthy K, Laskowitz DT (2016) Cellular and molecular mechanisms of secondary neuronal injury following traumatic brain injury. Transl Res Trauma Brain Injury, CRC Press/Taylor and Francis Group / Boca Raton (FL), Chapter 5. https://doi.org/10.1201/b18959-10
Guerriero RM, Giza CC, Rotenberg A (2015) Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep 15: 1–11. https://doi.org/10.1007/s11910-015-0545-1
Hinzman JM, Thomas TC, Quintero JE, Gerhardt GA, Lifshitz J (2012) Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J Neurotrauma 29: 1197–1208. https://doi.org/10.1089/neu.2011.2261
Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST (1993) Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem 61: 2015–2024. https://doi.org/10.1111/j.1471-4159.1993.tb07437.x
Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C (2010) Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113: 564–570. https://doi.org/10.3171/2009.12.jns09689
Lakshmanan R, Loo JA, Drake T, Leblanc J, Ytterberg AJ, McArthur DL, Etchepare M, Vespa PM (2010) Metabolic crisis after traumatic brain injury is associated with a novel microdialysis proteome. Neurocrit Care 12: 324–336. https://doi.org/10.1007/s12028-010-9342-5
Aiba I, Shuttleworth CW (2012) Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J Physiol 590: 5877–5893. https://doi.org/10.1113/jphysiol.2012.234476
Orrenius S, Gogvadze V, Zhivotovsky B (2015) Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun 460: 72–81. https://doi.org/10.1016/j.bbrc.2015.01.137
Sattler R, Tymianski M (2001) Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol 24: 107–129. https://doi.org/10.1385/mn:24:1-3:107
Balu R (2014) Inflammation and immune system activation after traumatic brain injury. Curr Neurol Neurosci Rep 14: 1–8. https://doi.org/10.1007/s11910-014-0484-2
Lehnardt S (2010) Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 58: 253–263. https://doi.org/10.1002/glia.20928
Karve IP, Taylor JM, Crack PJ (2016) The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol 173: 692–702. https://doi.org/10.1111/bph.13125
Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI (2014) Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol 73: 14–29. https://doi.org/10.1097/nen.0000000000000021
Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275: 305–315. https://doi.org/10.1016/j.expneurol.2015.03.020
Landeghem FKV, Weiss T, Oehmichen M, Deimling AV (2006) Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J Neurotrauma 23: 1518–1528. https://doi.org/10.1089/neu.2006.23.1518
Das M, Mohapatra S, Mohapatra SS (2012) New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammat 9: 1–12. https://doi.org/10.1186/1742-2094-9-236
Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK (1996) Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Mol Brain Res 36: 287–291. https://doi.org/10.1016/0169-328x(95)00274-v
Chio C-C, Chang C-H, Wang C-C, Cheong C-U, Chao C-M, Cheng B-C, Yang C-Z, Chang C-P (2013) Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-α. BMC Neurosci 14: 1–12. https://doi.org/10.1186/1471-2202-14-33
Lu K-T, Wang Y-W, Yang J-T, Yang Y-L, Chen H-I (2005) Effect of interleukin-1 on traumatic brain injury–induced damage to hippocampal neurons. J Neurotrauma 22: 885–895. https://doi.org/10.1089/neu.2005.22.885
Shohami E, Bass R, Wallach D, Yamin A, Gallily R (1996) Inhibition of tumor necrosis factor alpha (TNFα) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab 16: 378–384. https://doi.org/10.1097/00004647-199605000-00004
Babchenko VY, Belova AS, Bashirzade AA, Tikhonova MA, Demin KA, Zabegalov KN, Petersen EV, Kalueff AV, Amstislavskaya TG (2022) Traumatic brain injury models in zebrafish (Danio rerio). Neurosci Behav Physiol 52: 405–414. https://doi.org/10.1089/zeb.2012.0777
Zulazmi NA, Arulsamy A, Ali I, Zainal Abidin SA, Othman I, Shaikh MF (2021) The utilization of small non-mammals in traumatic brain injury research: A systematic review. CNS Neurosci Ther 27: 381–402. https://doi.org/10.1111/cns.13590
Westerfield M (2007) The Zebrafish Book. A guide for the laboratory use of zebrafish (Danio rerio).
Schmidt R, Beil T, Strähle U, Rastegar S (2014) Stab wound injury of the zebrafish adult telencephalon: a method to investigate vertebrate brain neurogenesis and regeneration. J Vis Exp 90: e51753. https://doi.org/10.3791/51753
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90: 54–58. https://doi.org/10.7287/peerj.preprints.1718v2
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205: 38–44. https://doi.org/10.1016/j.bbr.2009.06.022
Cleal M, Fontana BD, Ranson DC, McBride SD, Swinny JD, Redhead ES, Parker MO (2021) The Free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behav Res Methods 53: 536–557. https://doi.org/10.3758/s13428-020-01452-x
Fontana BD, Cleal M, Clay JM, Parker MO (2019) Zebrafish (Danio rerio) behavioral laterality predicts increased short-term avoidance memory but not stress-reactivity responses. Anim Cogn 22: 1051–1061. https://doi.org/10.1007/s10071-019-01296-9
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 25: 402–408. https://doi.org/10.1006/meth.2001.1262
Alburges ME, Narang N, Wamsley JK (1993) A sensitive and rapid HPLC-ECD method for the simultaneous analysis of norepinephrine, dopamine, serotonin and their primary metabolites in brain tissue. Biomed Chromatogr 7: 306–310. https://doi.org/10.1002/bmc.1130070605
Fontana BD, Cleal M, Gibbon AJ, McBride SD, Parker MO (2021) The effects of two stressors on working memory and cognitive flexibility in zebrafish (Danio rerio): the protective role of D1/D5 agonist on stress responses. Neuropharmacology 196: 108681. https://doi.org/10.1016/j.neuropharm.2021.108681
Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, Duckworth JL, Head BP (2017) Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell Mol Neurobiol 37: 571–585. https://doi.org/10.1007/s10571-016-0400-1
Ladak AA, Enam SA, Ibrahim MT (2019) A review of the molecular mechanisms of traumatic brain injury. World Neurosurg 131: 126–132. https://doi.org/10.1016/j.wneu.2019.07.039
Akamatsu Y, Hanafy KA (2020) Cell death and recovery in traumatic brain injury. Neurotherapeutics 17: 446–456. https://doi.org/10.1007/s13311-020-00840-7
Clark RS, Kochanek PM, Chen M, Watkins SC, Marion DW, Chen J, Hamilton RL, Loeffert JE, Graham SH (1999) Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J 13: 813–821. https://doi.org/10.1097/00008506-200007000-00019
Dressler J, Hanisch U, Kuhlisch E, Geiger KD (2007) Neuronal and glial apoptosis in human traumatic brain injury. Int J Legal Med 121: 365–375. https://doi.org/10.1007/s00414-006-0126-6
Ng I, Yeo T-T, Tang W-Y, Soong R, Ng P-Y, Smith DR (2000) Apoptosis occurs after cerebral contusions in humans. Neurosurgery 46: 949–956. https://doi.org/10.1227/00006123-200004000-00034
Zhang X, Graham SH, Kochanek PM, Marion DW, Nathaniel PD, Watkins SC, Clark RS (2003) Caspase-8 expression and proteolysis in human brain after severe head injury. FASEB J 17: 1367–1369. https://doi.org/10.1096/fj.02-1067fje
Zhang X, Alber S, Watkins SC, Kochanek PM, Marion DW, Graham SH, Clark RS (2006) Proteolysis consistent with activation of caspase-7 after severe traumatic brain injury in humans. J Neurotrauma 23: 1583–1590. https://doi.org/10.1089/neu.2006.23.1583
McIlwain DR, Berger T, Mak TW (2015) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 7: 1–15. https://doi.org/10.1101/cshperspect.a008656
Salvesen GS, Dixit VM (1999) Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A 96: 10964–10967. https://doi.org/10.1073/pnas.96.20.10964
Kenny EM, Fidan E, Yang Q, Anthonymuthu TS, New LA, Meyer EA, Wang H, Kochanek PM, Dixon CE, Kagan VE (2019) Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit Care Med 47: 410. https://doi.org/10.1097/ccm.0000000000003555
Zhang L, Wang H (2018) Autophagy in traumatic brain injury: a new target for therapeutic intervention. Front Mol Neurosci 11: 190. https://doi.org/10.3389/fnmol.2018.00190
Zhao P, Li C, Chen B, Sun G, Chao H, Tu Y, Bao Z, Fan L, Du X, Ji J (2020) Up-regulation of CHMP4B alleviates microglial necroptosis induced by traumatic brain injury. J Cell Mol Med 24: 8466–8479. https://doi.org/10.1111/jcmm.15406
Chaoul V, Awad M, Harb F, Najjar F, Hamade A, Nabout R, Soueid J (2022) Saffron Extract Attenuates Anxiogenic Effect and Improves Cognitive Behavior in an Adult Zebrafish Model of Traumatic Brain Injury. Int J Mol Sci 23: 11600. https://doi.org/10.3390/ijms231911600
McCutcheon V, Park E, Liu E, Sobhebidari P, Tavakkoli J, Wen X-Y, Baker AJ (2017) A novel model of traumatic brain injury in adult zebrafish demonstrates response to injury and treatment comparable with mammalian models. J Neurotrauma 34: 1382–1393. https://doi.org/10.1089/neu.2016.4497
Tikhonova MA, Maslov NA, Bashirzade AA, Nehoroshev EV, Babchenko VY, Chizhova ND, Tsibulskaya EO, Akopyan AA, Markova EV, Yang Y-L (2022) A novel laser-based zebrafish model for studying traumatic brain injury and its molecular targets. Pharmaceutics 14: 1751. https://doi.org/10.3390/pharmaceutics14081751
Chou A, Krukowski K, Jopson T, Zhu PJ, Costa-Mattioli M, Walter P, Rosi S (2017) Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc Natl Acad Sci U S A 114: E6420–E6426. https://doi.org/10.1073/pnas.1707661114
Fujimoto ST, Longhi L, Saatman KE, McIntosh TK (2004) Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev 28: 365–378. https://doi.org/10.1016/j.neubiorev.2004.06.002
Zohar O, Schreiber S, Getslev V, Schwartz J, Mullins P, Pick C (2003) Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118: 949–955. https://doi.org/10.1016/s0306-4522(03)00048-4
Mirzalieva O, Juncker M, Schwartzenburg J, Desai S (2022) ISG15 and ISGylation in human diseases. Cells 11: 538. https://doi.org/10.3390/cells11030538
Nakka VP, Mohammed AQ (2020) A critical role for ISGylation, ubiquitination and, SUMOylation in brain damage: implications for neuroprotection. Neurochem Res 45: 1975–1985. https://doi.org/10.1007/s11064-020-03066-3
Kang JA, Kim YJ, Jeon YJ (2022) The diverse repertoire of ISG15: More intricate than initially thought. Exp Mol Med 54: 1779–1792. https://doi.org/10.1038/s12276-022-00872-3
Demirci Y, Cucun G, Poyraz YK, Mohammed S, Heger G, Papatheodorou I, Ozhan G (2020) Comparative transcriptome analysis of the regenerating zebrafish telencephalon unravels a resource with key pathways during two early stages and activation of wnt/β-catenin signaling at the early wound healing stage. Front Cell Dev Biol 8: 584604. https://doi.org/10.3389/fcell.2020.584604
Kanagaraj P, Chen JY, Skaggs K, Qadeer Y, Connors M, Cutler N, Richmond J, Kommidi V, Poles A, Affrunti D (2020) Microglia stimulate zebrafish brain repair via a tumor necrosis factor-α-initiated inflammatory cascade. BioRXiv 2020–10. https://doi.org/10.1101/2020.10.08.330662
Yin G, Du M, Li R, Li K, Huang X, Duan D, Ai X, Yao F, Zhang L, Hu Z (2018) Glia maturation factor beta is required for reactive gliosis after traumatic brain injury in zebrafish. Exp Neurol 305: 129–138. https://doi.org/10.1016/j.expneurol.2018.04.008
Knoblach SM, Fan L, Faden AI (1999) Early neuronal expression of tumor necrosis factor-α after experimental brain injury contributes to neurological impairment. J Neuroimmunol 95: 115–125. https://doi.org/10.1016/s0165-5728(98)00273-2
Glushakova OY, Glushakov AO, Borlongan CV, Valadka AB, Hayes RL, Glushakov AV (2018) Role of caspase-3-mediated apoptosis in chronic caspase-3-cleaved tau accumulation and blood–brain barrier damage in the corpus callosum after traumatic brain injury in rats. J Neurotrauma 35: 157–173. https://doi.org/10.1089/neu.2017.4999
Kaneko Y, Tajiri N, Yu S, Hayashi T, Stahl CE, Bae E, Mestre H, Franzese N, Rodrigues Jr A, Rodrigues MC (2012) Nestin overexpression precedes caspase-3 upregulation in rats exposed to controlled cortical impact traumatic brain injury. Cell Med 4: 55–63. https://doi.org/10.3727/215517912x639306
Ringger NC, Tolentino PJ, McKinsey DM, Pike BR, Wang KKW, Hayes RL (2004) Effects of injury severity on regional and temporal mRNA expression levels of calpains and caspases after traumatic brain injury in rats. J Neurotrauma 21: 829–841. https://doi.org/10.1089/0897715041526177
Schweitzer J, Löhr H, Filippi A, Driever W (2012) Dopaminergic and noradrenergic circuit development in zebrafish. Dev Neurobiol 72: 256–268. https://doi.org/10.1002/dneu.20911
Jenkins PO, Mehta MA, Sharp DJ (2016) Catecholamines and cognition after traumatic brain injury. Brain 139: 2345–2371. https://doi.org/10.1093/brain/aww128
Wang Q, Oyarzabal EA, Song S, Wilson B, Santos JH, Hong J-S (2020) Locus coeruleus neurons are most sensitive to chronic neuroinflammation-induced neurodegeneration. Brain Behav Immun 87: 359–368. https://doi.org/10.1016/j.bbi.2020.01.003
Bueno-Nava A, Montes S, DelaGarza-Montano P, Alfaro-Rodriguez A, Ortiz A, Gonzalez-Pina R (2008) Reversal of noradrenergic depletion and lipid peroxidation in the pons after brain injury correlates with motor function recovery in rats. Neurosci Lett 443: 32–36. https://doi.org/10.1016/j.neulet.2008.07.046
Bari BA, Chokshi V, Schmidt K (2020) Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease. Neural Regen Res 15: 1006. https://doi.org/10.4103/1673-5374.270297
Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M (2012) Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338: 1353–1356. https://doi.org/10.1126/science.1228773
Anand SK, Sahu MR, Mondal AC (2021) Induction of oxidative stress and apoptosis in the injured brain: potential relevance to brain regeneration in zebrafish. Mol Biol Rep 48: 5099–5108. https://doi.org/10.21203/rs.3.rs-549302/v1
Schmidt R, Strähle U, Scholpp S (2013) Neurogenesis in zebrafish–from embryo to adult. Neural Dev 8: 1–13. https://doi.org/10.1186/1749-8104-8-3
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова