Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 6, стр. 798-816
Электрическая эпидуральная стимуляция спинного мозга децеребрированной крысы
П. Ю. Шкорбатова 1, 2, В. А. Ляховецкий 1, О. В. Горский 1, 2, Н. В. Павлова 1, Е. Ю. Баженова 1, Д. С. Калинина 2, 3, П. Е. Мусиенко 1, 2, Н. С. Меркульева 1, *
1 Институт физиологии им. И.П. Павлова РАН
Санкт-Петербург, Россия
2 Санкт-Петербургский Государственный Университет
Санкт-Петербург, Россия
3 Научно-технологический университет “Сириус”
пгт Сириус, Краснодарский край, Россия
* E-mail: mer-natalia@yandex.ru
Поступила в редакцию 28.03.2023
После доработки 12.05.2023
Принята к публикации 15.05.2023
- EDN: WHJWUM
- DOI: 10.31857/S0869813923060092
Аннотация
Децеребрированные препараты часто используются в экспериментальной нейрофизиологии для изучения многоуровневых физиологических процессов. Для исследования локомоции в острых опытах традиционно используется модель децеребрированной кошки. Мы задались вопросом, возможно ли заменить ее при электрической эпидуральной стимуляции спинного мозга на модель децеребрированной крысы. На остром препарате 16-ти децеребрированных на преколликулярном уровне крыс линии Вистар изучены возможность вызова локомоции, а также тоническая активность мышц и мышечные вызванные потенциалы. У 10 проводили гистологический контроль уровня децеребрации. Квадрипедальную ходьбу удалось вызвать у пяти животных, бипедальную ходьбу задними конечностями – у одного животного; при этом параметры вызванной локомоции не зависят от степени повреждения substantia nigra. Тоническая активность и амплитуда сенсорного компонента вызванных потенциалов мышц задних конечностей (mm. tibialis anterior и gastrocnemius medialis) зависят от рострокаудального уровня децеребрации животных – они выше при повреждении черной субстанции (substantia nigra). Таким образом, рассматриваемая модель позволяет успешно исследовать тоническую активность мышц и вызванные мышечные потенциалы, однако использование этой модели при изучении контролируемой локомоции требует проведения дополнительных исследований.
ВВЕДЕНИЕ
Экспериментальная физиология подразумевает проведение опытов на животных. До начала XX в. моделями в различных исследованиях служили лягушки, обезьяны, кролики, кошки и собаки, а с начала XX в. – и грызуны [1]. При этом вклад различных уровней нервной системы в регуляцию физиологических процессов, как правило, изучался на редуцированных моделях – животных, декортицированных или децеребрированных на различных уровнях. Децеребрированные препараты до сих пор успешно применяются в нейробиологии [2–4]. Одним из преимуществ таких препаратов является возможность работы без анестезии [5], применение которой изменяет физиологические показатели работы организма: частоту дыхания и сердечных сокращений, кровяное давление [6], а также влияет на нейрональную активность [7].
В исследованиях локомоции традиционно, начиная с работ Тиле и Шеррингтона [8, 9], преимущественно использовались децеребрированные кошки [10]. Децеребрированный препарат, в отличие от интактной кошки, позволяет исследовать механизмы локомоции, управляемой спинальными и стволовыми нейронными сетями. Ходьба децеребрированной кошки в остром эксперименте более стабильна и хорошо координирована, чем у спинальной [11]. В настоящее время кошек все реже используют в качестве лабораторных животных по этическим соображениям [12] (тем не менее, см. работы [13, 14]). Хорошей альтернативой в таком случае могут стать децеребрированные препараты грызунов (крыс и мышей), обладающих рядом преимуществ, среди которых: бóльшая доступность животных, генетическая однородность линий, возможность использования трансгенных моделей [15].
Актуальность исследования обусловлена тем, что ряд методов посттравматического восстановления ходьбы у человека [16] направлен на модуляцию спинальных сетей, например, посредством электрической стимуляции спинного мозга, что диктует важность их изучения на животных моделях. Следует отметить, что степень восхождения спинного мозга крысы сходна с таковой у человека (сегменты спинного мозга крысы смещены относительно одноименных позвонков более рострально, чем у кошки). Кроме того, в отличие от кошки, которая является пальцеходящей, крыса, как и человек – стопоходящие [17]. Эти особенности могут быть существенны при выборе именно крысы в качестве животной модели для изучения спинальных нейронных сетей, а также трансляции в клиническую практику экспериментальных результатов применения электростимуляции спинного мозга.
Мы задались вопросом, возможно ли использование децеребрированных крыс в качестве замены кошек для исследования локомоторных сетей в острых экспериментах. Однако, в известных нам из литературных источников исследованиях локомоции широко используются преимущественно хронические спинальные крысы [17–20]. В отличие от спинальной, локомоторные способности децеребрированной крысы изучены лишь во время спонтанной ходьбы [21] или же при стимуляции мезэнцефалической локомоторной области (МЛО) [21–24]. Исследований ЭС поясничного утолщения для инициации локомоции на модели децеребрированной крысы до сих пор не проводилось.
Цель работы – изучение особенностей функционирования сенсомоторной системы децеребрированной крысы при электрической эпидуральной стимуляции (ЭС) поясничного утолщения спинного мозга в остром эксперименте. Практическая задача работы – оценка качества локомоции, потенциально возможной на модели децеребрированной крысы. Поскольку особенности локомоции кошки значительно зависят от уровня децеребрации [25], анализировали также зависимость различных показателей возбудимости спинальных и супраспинальных сетей крысы (тоническая активность, вызванные ответы мышц конечностей [26, 27]) от уровня децеребрации. Основное внимание уделялось активности задних конечностей как основной мишени ЭС поясничного утолщения.
МЕТОДЫ ИССЛЕДОВАНИЯ
Выборка. Опыты были выполнены на 16-и децеребрированных крысах, самцах линии Вистар массой тела 250–300 г. Экспериментальные процедуры проводились в соответствии с требованиями Директивы Совета Европы 2010/63EU Европейского парламента о защите животных, используемых в экспериментальных и других научных целях. Протокол исследования № 131-03-06 от 12 декабря 2022 г. был одобрен Этическим комитетом в области исследований на животных СПбГУ.
Хирургические процедуры. Животных наркотизировали смесью изофлурана (Laboratories Karizoo, S.A., Испания) на кислороде (4–5% для индукции и 1–2% для поддержания анестезии, скорость 0.8 л/мин, наркозно-дыхательный аппарат Зоомед, Россия). Производили билатеральную имплантацию биполярных миографических электродов в мышцы передних (двуглавую (mm. biceps brachii, Bi) и трехглавую (triceps brachii, Tr) мышцы плеча) и задних конечностей (переднюю большеберцовую (mm. tibialis anterior, TA) и медиальную икроножную (gastrocnemius medialis, GM) мышцы голени). Одному животному имплантировали электроды справа в большую приводящую (mm. adductor magnus, Add) и среднюю ягодичную (gluteus medius, Gl)) мышцы задних конечностей, а также в дорсальный аддуктор хвоста (m. abductor caudae dorsalis, ACD). Электроды представляли собой пару проводов из нержавеющей стали в тефлоновой изоляции (AS632, Cooner Wire, Chatsworth, CA, США) диаметром 0.15 и 0.3 мм (в зависимости от размера исследуемой мышцы) с участками без изоляции длиной 0.5 мм.
Перед выполнением децеребрации этилоном 4.0 (Ethicon, США) накладывали лигатуры на сонные артерии, затем голову крысы закрепляли в стереотаксис и производили разрез кожи головы по средней сагиттальной линии (рис. 1a). Далее удаляли надкостницу и выравнивали голову животного так, чтобы точка сращения затылочных и теменных костей (lambda) и точка сращения теменных и лобных костей (bregma) находились на горизонтальной линии (рис. 1b). При помощи микрощипцов Люэра выполняли билатеральную краниотомию, включающую удаление теменной и частично лобной костей, не затрагивая медиального шва между ними (рис. 1c). Для предотвращения кровотечения из переднего сагиттального синуса накладывали лигатуры этилоном 4.0 на краниальный и каудальный участки этих костей с подхватом синуса (рис. 1d). Далее костный мостик между лигатурами удаляли, как описано у Добсона и Харриса [28] (рис. 1e). После снятия твердой мозговой оболочки, кюреткой удаляли кору головного мозга так, чтобы открыть доступ к пластинке четверохолмия. Затем лезвием, закрепленным в держателе стереотаксиса под углом 65°–90°, выполняли отсечение части головного мозга рострально от передних холмиков (рис. 1f, g), эту часть затем удаляли при помощи шпателя. В полость черепа помещали ватные тампоны и, при необходимости, гемостатические губки. Температуру тела в процессе процедуры децеребрации поддерживали на уровне 34–35°C, как рекомендовано [29]. Вся процедура подготовки и собственно децеребрации занимала около 40 мин.
Рис. 1.
Методика проведения эксперимента. (a–g) Череп животного на различных этапах проведения децеребрации: (a) – местоположение разреза кожи и точек “lambda” и “bregma” на черепе, вид сверху; (b) – местоположение точек “lambda” и “bregma”, и горизонтальная линия между ними на черепе, вид сбоку; (с) – местоположение предполагаемых краниотомий (пунктиром) справа и слева относительно точек “lambda” и “bregma”; (d) – местоположение накладываемых лигатур (ligatures) и костный мостик (bone bridge) после выполнения краниотомий билатерально. (e) – Вид на поверхность коры головного мозга после удаления костного мостика. (f) – Положение децеребрационного среза относительно пластинки четверохолмия после удаления коры головного мозга, SC – передние холмики, IC – задние холмики; (g) – положение и угол децеребрационного среза относительно линии “лямбда–брегма”, вид сбоку; (h) – схема экспериментальной установки для регистрации вызванных потенциалов и вызова ходьбы.
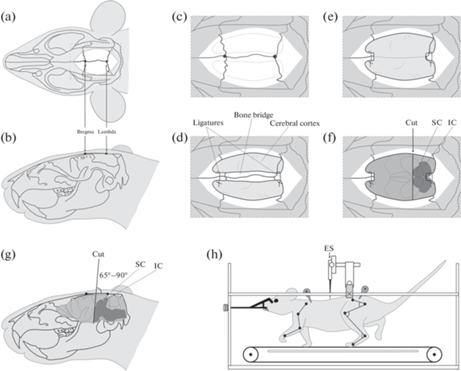
Гистологический контроль. После окончания эксперимента животных глубоко анестезировали смесью изофлурана на кислороде (4–5%) и проводили транскардиальную перфузионную фиксацию головного и спинного мозга 4% раствором параформальдегида (Merck, USA) на 0.1 М фосфатном буфере (Росмедбио, Россия). После вскрытия позвоночного канала определяли точное положение точек ЭС относительно сегментов спинного мозга. Затем извлекали головной мозг и помещали в 30% раствор сахарозы. Для определения рострокаудального уровня децеребрации (у 10 животных) на замораживающем микротоме изготавливали парасагиттальные срезы головного мозга толщиной 50 мкм, которые затем монтировали на предметные стекла и окрашивали 4% крезилвиолетом (рН 4.5) по методу Ниссля. При микроскопии срезов билатерально отмечали положение красных ядер (nucleus ruber) и черной субстанции (substantia nigra, SN), а также оценивали степень их повреждения.
Электрофизиологическое исследование. Для проведения электрофизиологических экспериментов была разработана экспериментальная установка (рис. 1h). Позвоночник крысы закрепляли зажимами в области поперечных отростков позвонков VТ11-VТ12 и VL4-VL5 в специально сконструированной раме. Производили ламинэктомию позвонков VТ13–VL2, чтобы открыть доступ к сегментам L1–S3 спинного мозга. ЭС дорсальной поверхности спинного мозга осуществляли прямоугольными бифазными импульсами монополярно (Model 2100, A-M Systems, Sequim, WA, США) при помощи серебряного шарикового электрода диаметром 0.5 мм. Индифферентный электрод, изготовленный из иглы 21G, располагался под кожей живота. За 20–30 мин до начала эксперимента анестезию отключали, а температуру тела животного повышали до 37°C [29]. Эксперимент начинали через 40–50 мин после проведения децеребрации.
Для вызова ходьбы передние и задние конечности крысы помещали на движущуюся ленту моторизованного тредбана. Рандомизированно осуществляли ЭС (5–40 Гц, 25–300 мкА) различных точек поверхности спинного мозга внутри ламинэктомии. Расстояние между соседними точками ЭС составляло 2 мм. Поскольку положение поясничных и крестцовых сегментов спинного мозга крысы относительно позвонков варьирует (см. различия положений сегментов в [30] (рис. 1) и [31] (рис. 1b, Supplement)), точное определение стимулируемого сегмента производили post-mortem на основании положения дорсальных корешков. К ЭС последующей точки переходили в случае получения стабильной квадрипедальной ходьбы в данной точке или же после длительного безуспешного перебора различных сочетаний частоты и силы тока ЭС. Эксперимент прекращали, если стабильную квадрипедальную ходьбу при переборе всех точек ЭС вызвать не удавалось, или при ухудшении состояния животного. Длительность эксперимента не превышала 9 ч.
При записи тонической активности и вызванных потенциалов передние и задние конечности крысы касались неподвижной ленты тредбана. Вызванные потенциалы мышц регистрировали при ЭС (частота 1 Гц, длительность импульса 0.2–0.3 мс, сила тока 10–100 мкА, шаг 10 мкА) сегмента L2. На каждом токе регистрировали 10 ответов. Для анализа вызванных потенциалов использовали среднюю амплитуду сенсорного компонента, выделенного на основе своей латентности (более 6 мс), ответов TA и GM, полученную на субмаксимальном токе, 70 мкА. На этом токе сенсорный компонент был выражен у большинства животных. Сигналы от ЭМГ электродов усиливались (Model 1700, A-M Systems, Sequim, WA, США) и оцифровывались с частотой дискретизации 20 кГц (LTR-EU-16, АЦП LTR11, L-Card, Москва, Россия).
Для анализа тонической активности ректифицировали и усредняли ЭМГ-активность на протяжении 3–5 с, в течение которых крыса была неподвижна. При локомоции ЭМГ активность фильтровали в диапазоне 100–2000 Гц, ректифицировали и усредняли в рамках цикла ходьбы, определяемого на основе покадрового анализа видео. Нормировка осуществлялась в рамках каждой мышцы каждого животного так, чтобы площадь под кривой ЭМГ составляла 100%. Обработка ЭМГ проводилась в пакете прикладных программ для решения задач технических вычислений MATLAB (Mathworks, Natick, MA, США) с помощью специализированных скриптов, разработанных авторами статьи.
Исследование кинематики. Чтобы охарактеризовать кинематику локомоторных движений задних конечностей, на гребне подвздошной кости, головке бедра, латеральном мыщелке бедра, латеральной стороне лодыжки и пятом плюсневом суставе были установлены маркеры, а вид сбоку (слева и справа) шагающей крысы был записан на видео (100 кадров/с). Для расчета длины и длительности шага, а также объема движения в суставах видеозаписи анализировались покадрово. Углы в тазобедренном, коленном и голеностопном суставах измеряли в тот момент, когда конечность была максимально согнута во время фазы переноса, и в момент, когда она была максимально вытянута в фазе опоры. Диапазоны изменения углов были рассчитаны как разность между этими значениями для каждого сустава.
Аналогично [32], для оценки качества межконечностной координации и последующего сравнения с известными значениями показателей локомоции децеребрированных кошек, рассчитывали коэффициенты асимметрии длины и периода шага. Лучшей межконечностной координации отвечает меньший коэффициент асимметрии. Коэффициент асимметрии длины шага вычисляли как: $\left| {{{S}_{{{\text{left}}}}} - {{S}_{{{\text{right}}}}}} \right|{\text{/}}{{S}_{{{\text{left}}}}} + {{S}_{{{\text{right}}}}},$ где Sleft и Sright – длины шагов левой и правой конечности соответственно. Длина шага рассчитывалась как расстояние между положением лапы в начале и в конце фазы опоры. Коэффициент асимметрии периода шага рассчитывали аналогично: $\left| {{{T}_{{{\text{left}}}}} - {{T}_{{{\text{right}}}}}} \right|{\text{/}}{{T}_{{{\text{left}}}}} + {{T}_{{{\text{right}}}}},$ где Tleft и Tright – периоды шагов левой и правой конечности соответственно. От каждого животного для анализа брали одну пробу с минимальным значением асимметрии длительности шага.
Ранее мы выявили, что такие характеристики вызванной ЭС локомоции, как длительность задержки ходьбы (время от начала стимуляции до начала ходьбы) и выраженность постэффекта (длительность ходьбы после прекращения стимуляции) различны у разных видов локомоции [33]. Поэтому в данной работе мы также оценили эти характеристики.
Статистический анализ. Данные представлены как среднее значение ± стандартное отклонение. Статистическую значимость отличий распределений различных параметров в зависимости от степени повреждения SN оценивали с помощью непарного критерия Манна–Уитни на уровне p < 0.05 (объектом исследования являлась задняя конечность животного) с помощью программного обеспечения для статистической обработки данных Prism 7.0 (GraphPadSoftware, La Jolla, CA, США).
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
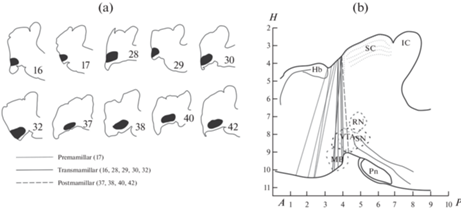
Гистологическое исследование. Согласно описанию процедуры децеребрации, у всех животных передние холмики были интактными билатерально (преколликулярный уровень децеребрационного среза). Гистологическое исследование парасагиттальных срезов мозга децеребрированных крыс, у которых была возможность взять гистологический материал (n = 10), показало, что у 4-х животных мамиллярные тела были сохранены (премамиллярный уровень), у 5-ти разрез проходил по мамиллярным телам (трансмамиллярный уровень) и у одного животного мамиллярные тела были удалены (постмамиллярный уровень). У всех крыс были интактными красные ядра; у части животных (6/10) была в разной степени повреждена SN (трансмамиллярный и постмамиллярный уровень), тогда как у остальных (4/10) она оставалась интактной (премамиллярный уровень). Примеры гистологических срезов мозга крыс с разной степенью повреждения SN, а также схема положения децеребрационного разреза у всех животных относительно структур головного мозга, представлены на рис. 2.
Рис. 2.
Положение децеребрационного разреза относительно черной субстанции (SN) и других структур мозга. (a) – Рисунки парасагиттальных срезов среднего мозга отдельных крыс после децеребрации, SN отмечена черным. (b) – Положение децеребрационных разрезов всех исследованных крыс на схеме среднего мозга. SC – Передние холмики, IC – задние холмики, RN – красное ядро, MB – мамиллярные тела, VTA – вентральная тегментальная область, Hb – хабенула, Pn – ножки мозжечка.
Общие особенности поведения децеребрированных крыс. Все децеребрированные крысы, как с постмамиллярной, так и с трансмамиллярной и премамиллярной перерезкой, обнаруживали высокую степень активности после децеребрации: они проявляли способность к спонтанным ритмическим локомоторно-подобным движениям конечностей, перемежающихся с периодами покоя, у них частично оставались способности к установочным рефлексам (периодически они поднимали голову и плечевой пояс). В периоды активности они отвечали вздрагиванием, поворотом головы или локомоторной активностью на звуки, прикосновения и ноцицептивные стимулы (уколы), даже в более выраженной степени, чем интактные животные. Этим была вызвана необходимость продолжать ингаляционную анестезию после проведения процедуры децеребрации, во время закрепления животного в стереотаксической раме. Некоторые животные начинали спонтанно ходить при движении ленты тредбана или прикосновении к плантарной поверхности стопы или хвосту.
Локомоция под действием ЭС. Из 16-ти крыс вызов локомоции при ЭС был возможен у 13-ти. У различных животных, а также в различных пробах у одного животного, число задействованных при локомоции конечностей было разным, то есть наблюдалась квадрипедальная, трипедальная (участвовали две передние конечности и одна задняя), бипедальная (участвовали две передних, две задних или одна передняя и одна задняя конечности) или унипедальная (одна задняя конечность) ходьба. Конечности, не принимающие участие в ходьбе, как правило,были согнуты и прижаты к телу. На рис. 3а указано число крыс, у которых удалось хотя бы в одной пробе вызвать локомоцию с максимальным числом движущихся конечностей. Например, если у животного в 10 пробах с различными параметрами ЭС была вызвана трипедальная ходьба, а в одной – квадрипедальная, то оно отнесено в столбец “квадрипедально”, в табл. 1 указаны параметры ЭС, необходимые для вызова именно квадрипедальной ходьбы. Из рисунка видно, что бипедальную ходьбу задними конечностями удалось вызвать у 1 из 16 крыс (6%), квадрипедальную – у 5 из 16 крыс (31%). Число задействованных конечностей (в дальнейшем, режим ходьбы) не зависело от того, какой сегмент спинного мозга стимулировали (табл. 1).
Рис. 3.
Ходьба, обусловленная эпидуральной стимуляцией. (a) – Число крыс, у которых удалось вызвать различные режимы ходьбы: no – отсутствие ходьбы, 1 hindlimb – одной задней конечностью, 1 hindlimb, 1 forelimb – одной передней и одной задней, two forelimbs – бипедальн передними, two hindlimbs – бипедально задними, tripedal – трипедально, quadripedally – квадрипедально. Для каждой крысы указан режим ходьбы с максимальным числом движущихся конечностей. (b) – ЭМГ мышц передних и задних конечностей, отвечающих преимущественно за флексию и экстензию, при квадрипедальной ходьбе крысы. Tr – triceps brachii, Bi – biceps brachii, TA – tibialis anterior, GM – gastrocnemius medialis. L – левая конечность, R – правая конечность, Stim – канал стимуляции, Step – периодичность фаз ходьбы, перенос (swing, белые прямоугольники) и опора (stance, черные прямоугольники). (c) – ЭМГ в цикле шага при квадрипедальной ходьбе или бипедальной ходьбе задними конечностями (n = 12 мышц). Среднее ± стандартное отклонение.
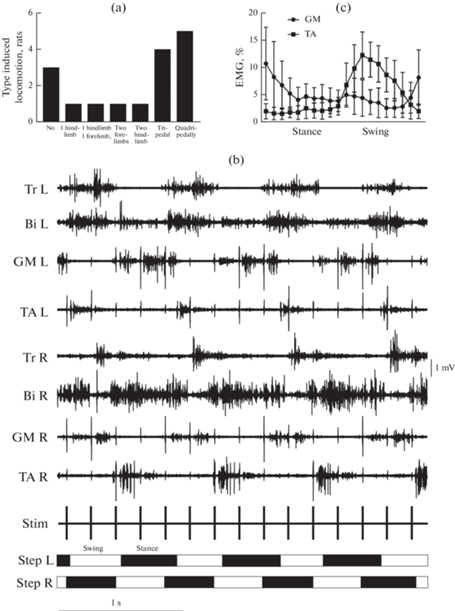
Таблица 1.
Эпидуральная стимуляция при попытках вызова ходьбы. Для каждого животного указаны параметры вызова режима ходьбы с максимальным числом движущихся конечностей
| № крысы | Стимуляция спинного мозга | Режим ходьбы | ||
|---|---|---|---|---|
| сегмент | частота, Гц | сила тока, мкА | ||
| 16 | L5 | 5 | 20–120 | Нет |
| 17 | L3 | 3 | 180 | Квадрипедальная |
| 18 | S1 | 40 | 100 | Трипедальная |
| 25 | L3 | 20–40 | 100–300 | Нет |
| 28 | L1 | 5 | 190 | Трипедальная |
| 29 | L2–L4, L6, S3 | 5 | 100–200 | Квадрипедальная |
| 30 | L6 | 5 | 90 | 1 задняя, 1 передняя |
| 32 | L6-S1 | 20 | 25 | Бипедальная задние |
| 33 | L4 | 5 | 70 | Унипедальная |
| 34 | L3 | 10 | 40 | Бипедально передние |
| 35 | L3, L6 | 20 | 90 | Трипедальная |
| 36 | L4 | 5–20 | 20–380 | Нет |
| 37 | L4 | 5 | 90–100 | Квадрипедальная |
| 38 | L6 | 20 | 70 | Квадрипедальная |
| 40 | L5–L6 | 5 | 50–80 | Квадрипедальная |
| 42 | L3–L4 | 5 | 300 | Трипедальная |
Следует отметить, что некоторые животные, у которых не удавалось вызвать квадрипедальную ходьбу при помощи ЭС, были способны к спонтанной координированной квадрипедальной ходьбе. Также отметим, что для разных животных использовали разную частоту ЭС: часть крыс могли ходить максимальным числом конечностей на более низких частотах (3–5 Гц, 69%), другие – на более высоких (20–40 Гц, 31%). При этом четкой зависимости между локомоторными способностями и частотой стимуляции мы не выявили: как в пределах индивидуального животного, так и при групповом сравнении.
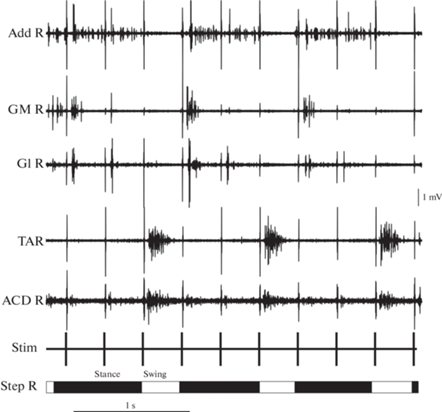
Пример работы мышц передних и задних конечностей при квадрипедальной ходьбе приведен на рис. 3b. Как можно видеть, контрлатеральные экстензоры (Tr, GM) передних и задних конечностей работают в фазе с испилатеральными флексорами (Bi, TA), в то время как ипсилатеральные экстензоры и флексоры работают, как правило, в противофазе, что свидетельствует о сохранности межконечностной координации. При квадрипедальной или бипедальной ходьбе задними конечностями в среднем активность экстензора GM имеет менее выраженный максимум в фазу опоры по сравнению с активностью флексора TA, которая имеет ярко выраженный максимум в фазу переноса (рис. 3c). У животного, у которого были имплантированы электроды в мышцы Add и Gl, в фазу опоры регистрировалась не только активность GM, но и этих двух мышц; также наблюдались ритмические движения хвоста (ритмическая активность ACD) (рис. 4). Выявленная согласованная работа мышц приводит к стабильной ходьбе. Однако такую координированную ходьбу, продолжающуюся значительное количество проб подряд, удалось вызвать лишь у двух животных.
Рис. 4.
ЭМГ мышц, предположительно отвечающих за постуральный контроль, при квадрипедальной ходьбе крысы. Add – adductor magnus, GM – gastrocnemius medialis, Gl – gluteus, TA – tibialis anterior, ACD – abductor caudae dorsalis. R – правая конечность, Stim – канал стимуляции, Step – периодичность фаз ходьбы, перенос (swing, белые прямоугольники) и опора (stance, черные прямоугольники).
Длительность задержки квадрипедальной ходьбы была крайне вариабельной и составляла от 0.5 с до 10 с для различных животных. Постэффект практически отсутствовал; после отключения ЭС одна крыса совершила 2 шага, а пять крыс полностью прекращали ходьбу.
Усредненные характеристики кинематики задних конечностей 6-ти крыс, у которых наблюдалась квадрипедальная ходьба или бипедальная ходьба обеих задних конечностей, представлены на рис. 5. Средняя длина шага составила 5.9 ± 2.4 см, средняя длительность – 0.8 ± 0.3 с (длительность переноса: 0.3 ± 0.1 с, длительность опоры: 0.5 ± 0.3 с). Асимметрия длины и длительности шага составили 0.15 ± 0.12 и 0.06 ± 0.05 соответственно. Наибольший объем движения наблюдался в голеностопном суставе (93° ± 35°), наименьший – в коленном суставе (13° ± 10°), объем движения в тазобедренном суставе составил 20° ± 14°.
Рис. 5.
Характеристики локомоции задних конечностей децеребрированной крысы при эпидуральной стимуляции. (a) Длина шага, см (n = 12 задних конечностей). (b) – Длительность фаз ходьбы, с: Swing – перенос, Stance – опора, Step – шаг (n = 12 задних конечностей). (c) – Объем движения в суставах: Hip – тазобедренный, Knee – коленный, Ankle – голеностоп (n = 12 задних конечностей). (d) – Асимметрия длины (Length) и длительности (Duration) шага (n = 6 пар задних конечностей).
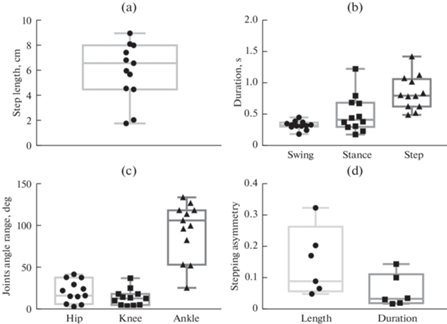
Зависимость характеристик ходьбы децеребрированной крысы от рострокаудального уровня децеребрации. Распределение крыс по режимам ходьбы не зависело от того, повреждена ли SN. И при поврежденной, и при интактной SN у ряда крыс удавалось вызвать квадрипедальную, а у иных – лишь унипедальную ходьбу (рис. 6g). ЭМГ активность TA и GM в цикле шага при би- или квадрипедальной ходьбе также не зависит от повреждения SN (рис. 6h, i). Между тем в группе крыс с поврежденной SN длительность шага выше, чем в группе крыс с интактной SN (0.66 ± 0.14 с и 1.05 ± ± 0.23 с соответственно, p < 0.01), за счет увеличения длительности опоры, которая составляет 0.64 ± 0.30 c, по сравнению с 0.31 ± 0.08 с у крыс с интактной SN (p < 0.01).
Рис. 6.
Влияние повреждения substancia nigra (SN) на сенсорный компонент вызванного ответа, тоническую активность мышц и режим ходьбы. (a) – Пример усредненных вызванных ответов при интактной (intact) и поврежденной (damaged) SN. Амплитуда сенсорного компонента вызванного ответа TA (b) и GM (c) при электрической эпидуральной стимуляции сегмента L2 (сила тока 70 мкА) при интактной (n = 8 мышц) и поврежденной (n = 12 мышц) SN. (d) – Пример тонической ЭМГ. Тоническая активность TA (e) и GM (f) при интактной (n = 8 мышц) и поврежденной (n = 12 мышц) SN. (g) – Число лап, активных при локомоции. ЭМГ в цикле шага TA (h) и GM (i) при квадрипедальной ходьбе или бипедальной ходьбе задними конечностями при интактной (n = 8 мышц) и поврежденной (n = 12 мышц) SN. Stance – опора, Swing – перенос, L – левая конечность, R – правая конечность, TA – tibialis anterior, GM – gastrocnemius medialis. Среднее ± Стандартное отклонение. * – p < 0.05
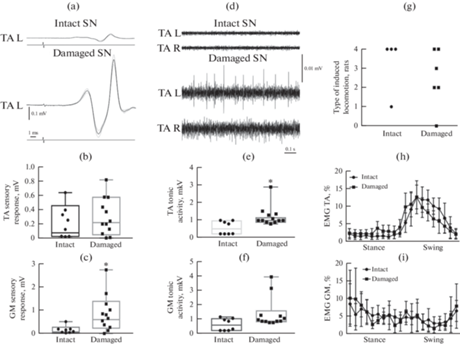
Вызванные мышечные потенциалы и тоническая активность. Амплитуда сенсорного компонента вызванных ответов TA и GM при стимуляции сегмента L2 (рис. 6a) у крыс с поврежденной SN несколько выше по сравнению с крысами с интактной SN (рис. 6b, 6c). У крыс с поврежденной SN амплитуда ответов TA составляет 0.30 ± 0.27 мВ, у крыс с интактной SN 0.21 ± 0.25 мВ (p = 0.63); в группе крыс с поврежденной SN амплитуда ответов GM составляет 0.85 ± 0.84 мВ, у крыс с интактной SN 0.15 ± 0.18 мВ (p = 0.03). Сходным образом, у крыс с поврежденной SN тоническая активность этих мышц (рис. 6d) выше, чем у крыс с интактной SN: как у TA (1.19 ± 0.62 и 0.58 ± 0.37 мкВ соответственно, p = 0.01), так и у GM (1.45 ± 1.15 и 0.66 ± ± 0.42 мкВ соответственно, p = 0.22) (рис. 6e, f).
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Общие особенности поведения децеребрированных крыс. Децеребрированные крысы рассматриваются как модель для изучения пищевого поведения [34], регуляции дыхания [29, 35], работы сердечно-сосудистой [36, 37] и мочевыделительной системы [38]. Однако при изучении локомоторной системы децеребрированный препарат крысы применяется не так широко, как препарат децеребрированной кошки [3–5, 39–42]. Это может быть связано с тем, что децеребрированные крысы обладают высоким уровнем спонтанной активности. Они совершают локомоторные движения и даже “попытки” бега [29], часто непредсказуемо реагируют на различные внешние раздражители, такие как укол [43] или прикосновение [44]. Высоким уровнем спонтанной активности эти препараты напоминают преколликулярный-премамиллярный препарат кошки [45], который способен к спонтанной локомоции при свободно движущейся ленте тредбана в остром эксперименте. Однако, как в нашей работе, так и в работах других авторов, спонтанную активность проявляли крысы не только с премамиллярной [36, 46], но и с постмамиллярной [21, 44] перерезкой. Отсутствие спонтанной активности у преколликулярно-постмамиллярно децеребрированной кошки делает ее моделью, более удобной для управления путем внешних воздействий, по сравнению с преколликулярно-постмамиллярной крысой. Тем не менее модель преколликулярно-постмамиллярной децеребрированной крысы позволила провести картирование мышечных сенсорных и моторных потенциалов, вызванных с помощью предложенного нами метода чрескостной стимуляции спинного мозга [47].
Отсутствие спонтанной ходьбы кошки связано с тем, что некоторые структуры головного мозга оказываются отсечены более каудальной плоскостью децеребрации. Между тем большая часть структур рассматриваемой зоны у крысы занимает приблизительно такое же, как у кошки, положение по отношению к плоскости среза. Немногими исключениями, согласно литературным данным, являются красные ядра и вентральная область покрышки (VTA) (см. [25, рис. 1; 48]). Гистологический анализ показал, что при используемых уровнях децеребрации крысы красные ядра, влияющие на активность флексоров передних и задних конечностей [49, 50], остаются интактными (рис. 2). Между тем красные ядра не влияют на возможность инициации локомоции как таковой. Напротив, VTA, которая, согласно некоторым литературным данным, отвечает за возникновение спонтанной локомоции [48], повреждается у части крыс выборки при проведении постмамиллярной и (у части крыс) трансмамиллярной децеребрации. Однако повреждение VTA незначительно влияет на их спонтанную активность, возможно, из-за небольшого объема этого повреждения (рис. 2). Результаты позволяют предполагать бо́льшую, по сравнению с кошкой, функциональную роль стволовых и мезэнцефальных структур головного мозга крысы в инициации и управлении локомоторной активностью.
Локомоция, обусловленная ЭС. При рассечении головного мозга на преколликулярном уровне, спинальная зона вызова ходьбы вперед с помощью ЭС у крыс столь же широка в рострокаудальном направлении (сегменты L2-S1, см. табл. 1), как и у кошек [32], но несколько смещена рострально, так как мотонейронные пулы задних конечностей у крысы расположены в более ростральных, чем у кошки, сегментах спинного мозга [51, 52]. Параметры ЭС для вызова ходьбы децеребрированных крыс и кошек [32] сходны (как правило, частота 5 Гц, сила тока 50–200 мкА, см. табл. 1). Также у этих двух моделей сходен период шагов.
Распределение децеребрированных крыс по режимам вызываемой ЭС ходьбы отличается от такового для децеребрированной кошки. Во-первых, наш собственный опыт показывает, что вызвать бипедальную ходьбу вперед задними конечностями у децеребрированной кошки значительно легче, чем у децеребрированной крысы (обеими задними конечностями ходило лишь 38% крыс, и то – если засчитывать единичные успешные попытки).
Во-вторых, при ЭС поясничного утолщения децеребрированные кошки, как правило, ходят бипедально [53], а не квадрипедально (квадрипедальная ходьба вызывается стимуляцией шейного отдела [53–55]). Напротив, среди способных к локомоции крыс, пять ходили квадрипедально и лишь одна – бипедально задними конечностями.
Структуры, частично ответственные за контроль локомоции передних конечностей (поля Фореля, субталамическое ядро) [56], расположены у крысы и у кошки приблизительно на одинаковом уровне по отношению к плоскостям децеребрации. Однако у кошки при премамиллярной децеребрации спонтанная квадрипедальная ходьба наблюдается менее, чем у половины животных [57]; при движении передних конечностей нередко заметно нарушение координации [58]. При более каудальных положениях плоскости децеребрации у кошки спонтанная локомоция отсутствует, а ходьбу передних конечностей, вызванную стимуляцией МЛО или движением ленты тредбана, возможно получить только после дополнительной спинализации препарата в нижнегрудном отделе [59, 60].
Известно, что восходящая проприоспинальная система значительно модулирует активность локомоторных сетей шейного утолщения [61, 62]. Одно из возможных объяснений вышеупомянутых феноменов – бóльшее возбуждающее влияние проприоспинальных волокон крысы по сравнению с кошкой, у которой при тех же уровнях децеребрации доминируют тормозные проприоспинальные влияния [59]. Принимая во внимание небольшой размер крыс, можно предположить, что у них во время ЭС происходила активация этих волокон, локализованных в пределах латерального канатика [63], и как следствие – активация шейного генератора шаганий.
В-третьих, наши собственные данные показывают, что при ЭС дорсальной поверхности спинного мозга децеребрированные кошки, как правило, не демонстрируют ходьбу вперед только одной задней конечностью; даже латеральное смещение электрода от центральной линии незначительно влияет на качество бипедальной ходьбы вперед [33], что отражает сохранность межконечностной координации. Унилатеральная ходьба возможна, например, при интраспинальной микростимуляции – у спинальных кошек [64, 65] или же при унилатеральной стимуляции дорсального корешка спинного нерва – у децеребрированных [66] или спинальных [65] кошек. Напротив, в настоящем исследовании, из 12 крыс, способных к локомоции задними конечностями, половина демонстрировали нарушения межконечностной координации: четыре крысы ходили трипедально, одна – унипедально и одна – одной задней и одной передней конечностями. Подобные данные получены и на хронических спинальных крысах во время ЭС [17]. Число спинальных крыс, способных к координированной ходьбе при ЭС сегмента L2, стимуляция которого наиболее часто приводила к вызову локомоции, составляло лишь 74%; остальные крысы при такой ЭС ходили унилатерально. Латеральное смещение электрода при ЭС хронических спинальных крыс также приводит к унипедальной ходьбе [18]. Предположительно полученные данные свидетельствует о большем вкладе в вызванную ЭС локомоцию децеребрированной крысы спинальных нейронных сетей, даже незначительный дисбаланс в активности которых приводит к нарушению межконечностной координации и локомоции в целом.
В пользу этого предположения также свидетельствует то, что и асимметрия длины, и асимметрия длительности шага децеребрированной крысы выше, чем у децеребрированной кошки [67]. Отметим, что у спинальных кошек асимметрия ходьбы при интраспинальной микростимуляции также выше, чем при ходьбе, обусловленной щипанием хвоста [65].
Важно отметить, что длительность задержки вызванной ЭС ходьбы крысы выше, а длительность пост-эффекта ниже, чем у кошки [33], что, вероятно, говорит о меньшей степени рекрутируемости спинальных сетей крысы в используемом экспериментальном дизайне.
Поддержание равновесия при локомоции, обусловленной ЭС. Объем движения в тазобедренном суставе у децеребрированной крысы был подобен объему движения интактной крысы, известному из литературных данных [68]. При этом объем движения в коленном суставе был ниже, а в голеностопе – выше, чем при ходьбе интактного животного [68]. Иной объем движения в суставах может быть связан с особенностями закрепления животного в стереотаксической раме, равно как и активность GM в фазе опоры, которая была выражена в меньшей степени, чем активность TA в фазу переноса. Однако при этом в фазу опоры наблюдалась активность мышц Add и Gl, подобная той, что наблюдается у интактной крысы [21]. Такая активность, присущая и децеребрированной кошке при незакрепленном тазе [40], может быть связана с тем, что крыса в нашем эксперименте фиксировалась в стереотаксической раме только за остистые отростки позвонков; таз при этом оставался свободным. Вместе с выявленной нами циклической активностью мышц хвоста (ACD), аналогичной функции хвоста у кошек [69] и собак [70], активность Add и Gl может использоваться для поддержания равновесия децеребрированной крысы при ходьбе.
Влияние ростро-каудального уровня децеребрации на характеристики модели крысы. Поскольку децеребрация неизбежно приводит к нарушению целостности ряда структур головного мозга, в частности тех, что отвечают за моторный контроль, мы сравнили локомоторные способности у двух групп: с поврежденной и интактной SN. Ретикулярная часть SN (SNr) оказывает модулирующее действие на активность локомоторных сетей (см. [22, 71, 72]). При повреждении SN вследствие преколликулярно-постмаммиллярной децеребрации вызов локомоции во время стимуляции МЛО у крысы [22] и кошки ([25], см. рис. 1, наиболее каудальная плоскость децеребрации) невозможен. Между тем другим авторам [21] удалось вызвать локомоцию крысы при таком же экспериментальном дизайне. Нам удалось инициировать локомоцию и при поврежденной SN, что, возможно, еще раз свидетельствует о большем вкладе спинальных нейронных сетей в локомоцию, обусловленную ЭС спинного мозга.
Мы также обнаружили разный уровень тонической активности мышц и амплитуды сенсорного компонента вызванного ответа мышц у животных с поврежденной и интактной SN. Более высокий уровень тонической активности и бóльшая амплитуда сенсорного компонента при повреждении SN хорошо согласуются с данными об увеличении как тонической активности [73], так и амплитуды ответа TA и GM, вызванного пассивным движением [74], при разрушении нигростриарных путей при инъекции оксидопамина в SN крысы.
Таким образом, для использования крысы, децеребрированной на преколликулярном уровне, в задаче изучения контролируемой локомоции необходимо проведение дополнительных исследований. При этом имеющаяся модель позволяет успешно исследовать общую тоническую активность мышц и их вызванные ответы.
Список литературы
Stahnisch FW (2010) Chapter 11: on the use of animal experimentation in the history of neurology. Handb Clin Neurol 95: 129–148. https://doi.org/10.1016/S0072-9752(08)02111-8
Whelan PJ (1996) Control of locomotion in the decerebrate cat. Prog Neurobiol 49: 481–515.
Domínguez-Rodríguez LE, Stecina K, García-Ramírez DL, Mena-Avila E, Milla-Cruz JJ, Martínez-Silva L, Zhang M, Hultborn H, Quevedo JN (2020) Candidate interneurons mediating the resetting of the locomotor rhythm by extensor group I afferents in the cat. Neuroscience 450: 96–112. https://doi.org/10.1016/j.neuroscience.2020.09.017
Aguilar Garcia IG, Dueñas-Jiménez JM, Castillo L, Osuna-Carrasco LP, De La Torre Valdovinos B, Castañeda-Arellano R, López-Ruiz JR, Toro-Castillo C, Treviño M, Mendizabal-Ruiz G, Duenas-Jimenez SH (2020) Fictive scratching patterns in brain cortex-ablated, midcollicular decerebrate, and spinal cats. Front Neural Circuits 27: 14. https://doi.org/10.3389/fncir.2020.00001
Silverman J., Garnett NL., Giszter SF, Heckman CJ, Kulpa-Eddy JA, Lemay MA, Perry CK, Pinter M (2005) Decerebrate mammalian preparations: unalleviated or fully alleviated pain? A review and opinion. Contempor Topics Lab Animal Sci 44: 34–36.
Sapru HN, Krieger AJ (1979) Cardiovascular and respiratory effects of some anesthetics in the decerebrate rat. Eur J Pharmacol 53: 151–158. https://doi.org/10.1016/0014-2999(79)90160-2
Ho SM, Waite PM (2002) Effects of different anesthetics on the paired-pulse depression of the h reflex in adult rat. Exp Neurol 177: 494–502. https://doi.org/10.1006/exnr.2002.8013
Thiele FH (1905) On the efferent relationship of the optic thalamus and Deiter’s nucleus to the spinal cord, with special reference to the cerebellar influx of Dr Hughlings Jackson and the genesis of the decerebrate rigidity of Ord and Sherrington. J Physiol 32: 358–384. https://doi.org/10.1113/jphysiol.1905.sp001089
Sherrington CS (1910) Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121. https://doi.org/10.1113/jphysiol.1910.sp001362
Frigon A (2020) Fundamental contributions of the cat model to the neural control of locomotion. In The Neural Control of Movement (P. 315–348). Academic Press.https://doi.org/10.1016/B978-0-12-816477-8.00013-2
Мусиенко ПЕ, Горский ОВ, Килимник ВА, Козловская ИБ, Courtine G, Edgerton VR, Герасименко ЮП (2013) Регуляция позы и локомоции у децеребрированных и спинализированных животных. Рос физиол журн им ИМ Сеченова 99: 392–405. [Musienko PE, Gorskii OV, Kilimnik VA, Kozlovskaia IB, Courtine G, Edgerton VR, Gerasimenko YuP (2013) Neuronal control of posture and locomotion in decerebrated and spinalized animals. Ross Fiziol Zh Im I M Sechenova 99: 392–405. (In Russ)].
Макарова МН (2021) Кошки в лабораторных исследованиях. Обзор литературы. Лабораторные животные для научных исследований. 1: 86–104. [Makarova MN (2021) Cats in laboratory studies. Literature review Laboratornyye Zhivotnyye Dlya Nauchnykh Issledovaniy. 1: 86–104. (In Russ)]. https://doi.org/10.29296/2618723X-2021-01-09
Harnie J, Audet J, Klishko AN, Doelman A, Prilutsky BI, Frigon A (2021) The spinal control of backward locomotion. J Neurosci 41: 630–647. https://doi.org/10.1523/JNEUROSCI.0816-20.2020
Fathi Y, Erfanian A (2022) Decoding bilateral hindlimb kinematics from cat spinal signals using three-dimensional convolutional neural network. Front Neurosci 16: 801818. https://doi.org/10.3389/fnins.2022.801818
Roussel M, Lemieux M, Bretzner F (2020) Using mouse genetics to investigate supraspinal pathways of the brain important to locomotion. In The Neural Control of Movement (P. 269–313). Academic Press. https://doi.org/10.1016/B978-0-12-816477-8.00012-0
Hofstoetter US, Freundl B, Binder H, Minassian K (2018) Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PloS One 13: e0192013. https://doi.org/10.1371/journal.pone.0192013
Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR (2005) Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 383: 339–344. https://doi.org/10.1016/j.neulet.2005.04.049
Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G, Micera S (2013) A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33: 19326–19340. https://doi.org/10.1523/JNEUROSCI.1688-13.2013
Wang S, Zhang LC, Fu HT, Deng JH, Xu GX, Li T, Ji XR, Tang PF (2021) Epidural electrical stimulation effectively restores locomotion function in rats with complete spinal cord injury. Neural Regen Res 16: 573–579. https://doi.org/10.4103/1673-5374.290905
Gerasimenko Y, Preston C, Zhong H, Roy RR, Edgerton VR, Shah PK (2019) Rostral lumbar segments are the key controllers of hindlimb locomotor rhythmicity in the adult spinal rat. J Neurophysiol 122: 585–600. https://doi.org/10.1152/jn.00810.2018
Nicolopoulos-Stournaras S, Iles JF (1984) Hindlimb muscle activity during locomotion in the rat (Rattus norvegicus) (Rodentia: Muridae). J Zool Lond 203: 427–440. https://doi.org/10.1111/j.1469-7998.1984.tb02342.x
Skinner RD, Garcia-Rill E (1984) The mesencephalic locomotor region (MLR) in the rat. Brain Res 323: 385–389. https://doi.org/10.1016/0006-8993(84)90319-6
Garcia-Rill E, Kinjo N, Atsuta Y, Ishikawa Y, Webber M, Skinner RD (1990) Posterior midbrain-induced locomotion. Brain Res Bull 24: 499–508. https://doi.org/10.1016/0361-9230(90)90103-7
Yang CT, Vaca L, Roy RR, Zhong H, Edgerton VR, Judy JW (2005) Neural-Ensemble activity of spinal cord L1/L2 during stepping in a decerebrate rat preparation. Conference Proceedings. 2nd Internat IEEE EMBS Conf Neural Engineer 66–69. https://doi.org/10.1109/CNE.2005.1419554
Grillner S, Shik ML (1973) On the descending control of the lumbosacral spinal cord from the “mesencephalic locomotor region”. Acta Physiol Scand 87: 320–333. https://doi.org/10.1111/j.1748-1716.1973.tb05396.x
Ivanenko YP, Gurfinkel VS, Selionov VA, Solopova IA, Sylos-Labini F, Guertin PA, Lacquaniti F (2017) Tonic and rhythmic spinal activity underlying locomotion. Curr Pharm Des 23: 1753–1763. https://doi.org/10.2174/1381612823666170125152246
Vargas Luna JL, Brown J, Krenn MJ, McKay B, Mayr W, Rothwell JC, Dimitrijevic MR (2021) Neurophysiology of epidurally evoked spinal cord reflexes in clinically motor-complete posttraumatic spinal cord injury. Exp Brain Res 239: 2605–2620. https://doi.org/10.1007/s00221-021-06153-1
Dobson KL, Harris J (2012) A detailed surgical method for mechanical decerebration of the rat. Exp Physiol 97: 693–698. https://doi.org/10.1113/expphysiol.2012.064840
Ghali GZ, Ghali MGZ (2020) Microneurosurgical techniques and perioperative strategies utilized to optimize experimental supracollicular decerebration in rats. J Integ Neurosci 19: 137–177. https://doi.org/10.31083/j.jin.2020.01.1153
Gilerovich EG, Moshonkina TR, Fedorova EA, Shishko TT, Pavlova NV, Gerasimenko YP, Otellin VA (2008) Morphofunctional characteristics of the lumbar enlargement of the spinal cord in rats. Neurosci Behav Physiol 38: 855–860. https://doi.org/10.1007/s11055-008-9056-8
Wenger N, Moraud EM, Gandar J, Musienko P, Capogrosso M, Baud L, Le Goff CG, Barraud Q, Pavlova N, Dominici N, Minev IR, Asboth L, Hirsch A, Duis S, Kreider J, Mortera A, Haverbeck O, Kraus S, Schmitz F, DiGiovanna J, van den Brand R, Bloch J, Detemple P, Lacour SP, Bézard E, Micera S, Courtine G (2016) Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med 22: 138–145. https://doi.org/10.1038/nm.4025
Merkulyeva N, Veshchitskii A, Gorsky O, Pavlova N, Zelenin PV, Gerasimenko Y, Deliagina TG, Musienko P (2018) Distribution of spinal neuronal networks controlling forward and backward locomotion. J Neurosci 38: 4695–4707. https://doi.org/10.1523/JNEUROSCI.2951-17.2018
Merkulyeva N, Lyakhovetskii V, Gorskii O, Musienko P (2022) Differences in backward and forward treadmill locomotion in decerebrated cats. J Exp Biol 225: jeb244210. https://doi.org/10.1242/jeb.244210
Darling RA, Ritter S (2009) 2-Deoxy-D-glucose, but not mercaptoacetate, increases food intake in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 297: R382–R386. https://doi.org/10.1152/ajpregu.90827.2008
Zhou D, Huang Q, Fung ML, Li A, Darnall RA, Nattie EE, St John WM (1996) Phrenic response to hypercapnia in the unanesthetized, decerebrate, newborn rat. Respir Physiol 104: 11–22. https://doi.org/10.1016/0034-5687(95)00098-4
Bedford TG, Loi PK, Crandall CC (1985) A model of dynamic exercise: the decerebrate rat locomotor preparation. J Appl Physiol 72: 121–127. https://doi.org/10.1152/jappl.1992.72.1.121
Ghali MGZ (2021) Dynamic changes in arterial pressure following high cervical transection in the decerebrate rat. J Spinal Cord Med 44: 399–410. https://doi.org/10.1080/10790268.2019.1639974
Yoshiyama M, Roppolo JR, Takeda M, de Groat WC (2013) Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am J Physiol Renal Physiol 304: F390–F396. https://doi.org/10.1152/ajprenal.00574.2012
Asanome M, Matsuyama K, Mori S (1998) Augmentation of postural muscle tone induced by the stimulation of the descending fibers in the midline area of the cerebellar white matter in the acute decerebrate cat. Neurosci Res 30: 257–269.
Musienko P, Courtine G, Tibbs JE, Kilimnik V, Savochin A, Garfinkel A, Roy RR, Edgerton VR, Gerasimenko Y (2012) Somatosensory control of balance during locomotion in decerebrated cat. J Neurophysiol 107: 2072–2082. https://doi.org/10.1152/jn.00730.2011
Musienko PE, Zelenin PV, Lyalka VF, Gerasimenko YP, Orlovsky GN, Deliagina TG (2012) Spinal and supraspinal control of the direction of stepping during locomotion. J Neurosci 32: 17442–17453. https://doi.org/10.1523/JNEUROSCI.3757-12.2012
Musienko PE, Deliagina TG, Gerasimenko YP, Orlovsky GN, Zelenin PV (2014) Limb and trunk mechanisms for balance control during locomotion. J Neurosci 2014. 34: 5704–5716. https://doi.org/10.1523/JNEUROSCI.4663-13.2014
Pickering AE, Paton JF (2006) A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J Neurosci Meth 155: 260–271. https://doi.org/10.1016/j.jneumeth.2006.01.011
Grill HJ, Norgren R (1978) Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res 14: 299–312. https://doi.org/10.1016/0006-8993(78)90570-x
Шик МЛ, Северин ФВ, Орловский ГН (1966) Управление ходьбой и бегом посредством электрической стимуляции среднего мозга. Биофизика XI: 659–666. [Shik ML, Severin FV, Orlovsky GN (1966) Control of walking and running by means of electric stimulation of the midbrain. Biophyzica XI: 659–666. (In Russ)].
Fouad K, Pearson KG (1997) Effects of extensor muscle afferents on the timing of locomotor activity during walking in adult rats. Brain Res 749: 320–328. https://doi.org/10.1016/S0006-8993(96)01328-5
Shkorbatova P, Lyakhovetskii V, Pavlova N, Popov A, Bazhenova E, Kalinina D, Gorskii O, Musienko P (2020) Mapping of the spinal sensorimotor network by transvertebral and transcutaneous spinal cord stimulation. Front Systems Neurosci 14: 555593. https://doi.org/10.3389/fnsys.2020.555593
Ishii K, Asahara R, Komine H, Liang N, Matsukawa K (2020) Pivotal role of the ventral tegmental area in spontaneous motor activity and concomitant cardiovascular responses in decerebrate rats. Brain Res 1729: 146616. https://doi.org/10.1016/j.brainres.2019.146616
Orlovsky GN (1972) Activity of rubrospinal neurons during locomotion. Brain Res 46: 99–112. https://doi.org/10.1016/0006-8993(72)90008-x
Basile GA, Quartu M, Bertino S, Serra MP, Boi M, Bramanti A, Anastasi GP, Milardi D, Cacciola A (2021) Red nucleus structure and function: from anatomy to clinical neurosciences. Brain Struct Funct 226: 69–91. https://doi.org/10.1007/s00429-020-02171-x
Nicolopoulos-Stournaras S, Iles JF (1983) Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol 217: 75–85. https://doi.org/10.1002/cne.902170107
Vanderhorst VG, Holstege G (1997) Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76. https://doi.org/10.1002/(sici)1096-9861(19970526)
Lavrov I, Musienko PE, Selionov VA, Zdunowski S, Roy RR, Edgerton VR, Gerasimenko Y (2015) Activation of spinal locomotor circuits in the decerebrated cat by spinal epidural and/or intraspinal electrical stimulation. Brain Res 1600: 84–92. https://doi.org/10.1016/j.brainres.2014.11.003
Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD (1992) Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull 28: 99–105. https://doi.org/10.1016/0361-9230(92)90235-p
Bogacheva IN, Musienko PE, Shcherbakova NA, Moshonkina TR, Savokhin AA, Gerasimenko YuP (2014) Analysis of locomotor activity in decerebrate cats using electromagnetic and epidural electrical stimulation of the spinal cord. Neurosci Behav Physi 44: 552–559. https://doi.org/10.1007/s11055-014-9950-1
Waller WH (1940) Progression movements elicited by subthalamic stimulation. J Neurophysiol 3: 300–307.
Garcia-Rill E, Skinner RD, Fitzgerald JA (1983) Activity in the mesencephalic locomotor region during locomotion. Exp Neurol 82: 609–622. https://doi.org/10.1016/0014-4886(83)90084-5
Орловский ГН (1969) Спонтанная и вызванная локомоция таламической кошки. Биофизика XIV: 1095–1102. [Orlovsky GN (1969) Spontaneous and evoked locomotion of the thalamic cat. Biophyzica XIVI: 1095–1102. (In Russ)].
Будакова НН, Шик МЛ (1974) Шагательные движения передних конечностей и феномен Шиффа–Шеррингтона. Бюлл экспер биол мед 77: 6–10. [Budakova NN, Shik ML (1974) Stepping movements of the forelimbs and Shiff-Sherrington phenomen. Byull eksper biol med 77: 6–10. (In Russ)].
Yamaguchi T (1992) Muscle activity during forelimb stepping in decerebrate cats. Jpn J Physiol 42: 489–399. https://doi.org/10.2170/jjphysiol.42.489
Juvin L, Simmers J, Morin D (2005) Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci 25: 6025–6035. https://doi.org/10.1523/JNEUROSCI.0696-05.2005
Reed WR, Shum-Siu A, Onifer SM, Magnuson DSK (2006) Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience 142: 1195–1207. https://doi.org/10.1016/j.neuroscience.2006.07.017
Miller S, Reitsma DJ, van der Meché FG (1973) Functional organization of long ascending propriospinal pathways linking lumbo-sacral and cervical segments in the cat. Brain Res 62: 169–188. https://doi.org/10.1016/0006-8993(73)90626-4
Barthélemy D, Leblond H, Provencher J, Rossignol S (2006) Non-locomotor and locomotor hindlimb responses evoked by electrical microstimulation of the lumbar cord in spinalized cats. J Neurophysiol 96: 3273–3292. https://doi.org/10.1152/jn.00203.2006
Barthélemy D, Leblond H, Rossignol S (2007) Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J Neurophysiol 97: 1986–2000. https://doi.org/10.1152/jn.00818.2006
Будакова НН (1971) Шагательные движения, вызываемые у мезэнцефалической кошки ритмическим раздражением дорсального корешка. Физиол журн СССР им ИМ Сеченова LVII: 1632–1640. [Budakova NN (1971) Stepping movements evoked by a rhythmic stimulation of a dorsal root in mesencephalic cat. Fiziol zhurnal SSSR im IM Sechenova LVII: 1632–1640. (In Russ)].
Lyakhovetskii V, Merkulyeva N, Gorskii O, Musienko P (2021) Simultaneous bidirectional hindlimb locomotion in decerebrate cats. Sci Rep 11: 3252. https://doi.org/10.1038/s41598-021-82722-2
Thota AK, Watson SC, Knapp E, Thompson B, Jung R (2005) Neuromechanical control of locomotion in the rat. J Neurotrauma 22: 442–465. https://doi.org/10.1089/neu.2005.22.442
Walker C, Vierck CJ Jr, Ritz LA (1998) Balance in the cat: role of the tail and effects of sacrocaudal transection. Behav Brain Res 91: 41–47. https://doi.org/10.1016/s0166-4328(97)00101-0
Wada N, Hori H, Tokuriki M (1993) Electromyographic and kinematic studies of tail movements in dogs during treadmill locomotion. J Morphol 217: 105–113. https://doi.org/10.1002/jmor.1052170109
Takakusaki K (2013) Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord 28: 1483–1491. https://doi.org/10.1002/mds.25669
de Vrind VAJ, van 't Sant LJ, Rozeboom A, Luijendijk-Berg MCM, Omrani A, Adan RAH (2021) Leptin receptor expressing neurons in the substantia nigra regulate locomotion, and in the ventral tegmental area motivation and feeding. Front Endocrinol 12: 680494. https://doi.org/10.3389/fendo.2021.680494
Double KL, Crocker AD (1993) Quantitative electromyographic changes following modification of central dopaminergic transmission. Brain Res 604(1–2): 342–344. https://doi.org/10.1016/0006-8993(93)90388-4
Wolfarth S, Konieczny J, Smiałowska M, Schulze G, Ossowska K (1996) Influence of 6-hydroxydopamine lesion of the dopaminergic nigrostriatal pathway on the muscle tone and electromyographic activity measured during passive movements. Neuroscience 74: 985–996. https://doi.org/10.1016/0306-4522(96)00418-6
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова


