Зоологический журнал, 2019, T. 98, № 4, стр. 377-386
Neotype Designations and Redescriptions of Dometorina taiwanica Tseng 1984 and Zygoribatula gratiosa tseng 1984 from Taiwan (Acari, Oribatida, Oripodoidea)
S. G. Ermilov 1, *, J.-R. Liao 2, **
1 Tyumen State University
625003 Tyumen, Russia
b National Taiwan University
10617 Taipei, Taiwan
* E-mail: ermilovacari@yandex.ru
** E-mail: d04632007@ntu.edu.tw
Поступила в редакцию 25.02.2018
После доработки 9.04.2018
Принята к публикации 28.04.2018
Аннотация
Two species of oribatid mites, Dometorina taiwanica Tseng 1984 (Scheloribatidae) and Zygoribatula gratiosa Tseng 1984 (Oribatulidae), are redescribed based on material collected from Taiwan; neotypes are designated for both species.
As we noted previously (Ermilov, Liao, 2017a; Liao et al., 2017), all type specimens of oribatid mites (Acari, Oribatida) described by Yi-Hsiung Tseng (1982, 1984) had been lost. Therefore, according to Article 75.3.4 of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999), a designation of neotypes is necessary.
During the taxonomic identification of oribatids from Taiwan, we have found enough specimens of Dometorina taiwanica Tseng 1984 (Scheloribatidae) and Zygoribatula gratiosa Tseng 1984 (Oribatulidae). Based on a close morphological similarity of collected specimens to the original descriptions (Tseng, 1984), we have designated a neotype for each of the species. The descriptions of Tseng are primitive and incomplete: texts that accompany the descriptions are very brief; figures are very small and generally unclear; the descriptions lack information about some morphological structures (e.g., leg setation, solenidia, morphology of gnathosoma) and do not provide their measurements. In sum, these shortcomings do not allow to identify these species clearly. Therefore, we present supplementary descriptions and summarize the main morphological traits which will help the identification of D. taiwanica and Z. gratiosa in the future.
This work is part of our study of the oribatid fauna of Taiwan (Ermilov, Liao, 2017, 2017a, 2017b).
METHODS
Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the ventral plate. Notogastral width refers to the maximum in dorsal aspect. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu–tibia–tarsus. Morphological terminology used in this paper follows that of F. Grandjean: see Travé and Vachon (1975) for references, Norton (1977) for leg setal nomenclature, and Norton and Behan-Pelletier (2009), for overview. Drawings were made with a camera lucida using a Leica transmission light microscope “Leica DM 2500”.
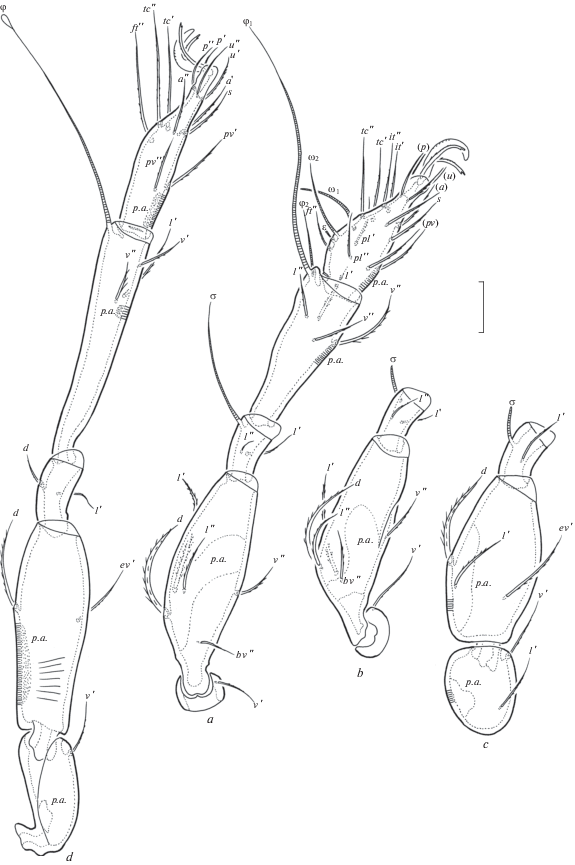
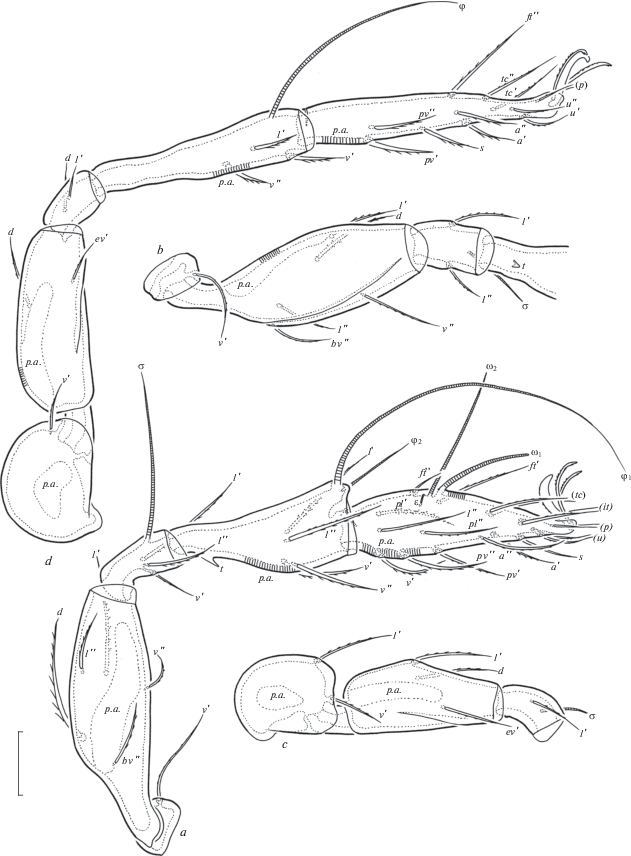
The following abbreviations are used: lam – lamella; tlam – translamella; plam – prolamella; slam – sublamella; Al – sublamellar porose area; ro, le, in, bs, ex – rostral, lamellar, interlamellar, bothridial and exobothridial setae, respectively; Ad – dorsosejugal porose area; D – dorsophragma; P – pleurophragma; cs – circumgastric scissure; csb – circumgastric sigillar band; pcar – pleural carina; nr – notogastral ridge; Sa, S1, S2, S3 – notogastral saccules; Aa, A1, A2, A3 – notogastral porose areas; c, da, la, dm, lm, dp, lp, h, p – notogastral setae; ia, im, ip, ih, ips – notogastral lyrifissures; gla – opisthonotal gland opening; Ap – postanal porose area; h, m, a – subcapitular setae; or – adoral seta; v, l, d, cm, acm, ul, sul, vt, lt – palp setae; ω – palp and leg solenidion; cha, chb – cheliceral setae; Tg – Trägårdh’s organ; Pd I, Pd II – pedotecta I, II, respectively; 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c – epimeral setae; dis – discidium; cp – circumpedal carina; g, ag, an, ad – genital, aggenital, anal and adanal setae, respectively; p.o. – preanal organ; iad – adanal lyrifissure; Tr, Fe, Ge, Ti, Ta – trochanter, femur, genu, tibia, tarsus, respectively; t – tooth; p.a. – porose area; σ, φ – leg solenidia; ɛ – leg famulus; v, ev, bv, l, d, ft, tc, it, p, u, a, s, pv, pl – leg setae.
The following abbreviations of collections are used: NTU – National Taiwan University, Taipei, Taiwan; SMNH – Senckenberg Museum of Natural History, Görlitz, Germany; TSUMZ – Tyumen State University Museum of Zoology, Tyumen, Russia.
NEOTYPE DESIGNATIONS
(Figs 1–3)
Fig. 1.
Dometorina taiwanica Tseng 1984, adult: a – dorsal view (legs not shown); b – ventral view (gnathosoma and legs not shown); c – subcapitulum, ventral view; d – palp, left, paraxial view; e) chelicera, left, paraxial view. Scale bar (µm): a, b – 50; c–e – 17.
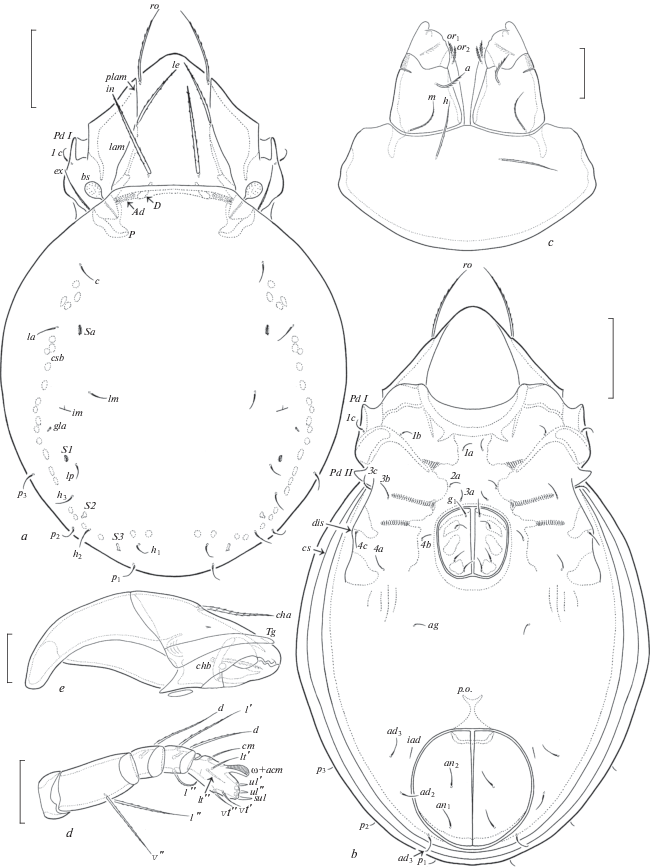
Fig. 2.
Dometorina taiwanica Tseng 1984, adult: a – lateral view (gnathosoma and legs not shown); b – posterior view. Scale bar 50 µm.
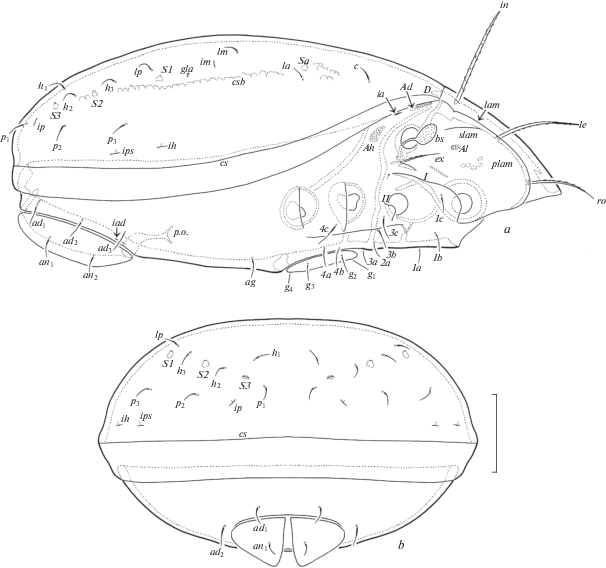
Fig. 3.
Dometorina taiwanica Tseng 1984, adult: a – leg I, right, antiaxial view; b – trochanter, femur and genu of leg II, right, antiaxial view; c – trochanter, femur and genu of leg III, left, antiaxial view; d – leg IV, right, paraxial view. Scale bar 20 µm.
Material examined. Neotype (♀) and 10 specimens (4 ♀♀, 6 ♂♂): locality #1, Taiwan, Qinbi, Beigan Township, Lienchiang County, leaves on Crossostephium chinense (Asteraceae), 5.VI.2017 (T. J. Lee).
Type deposition (all in ethanol with drop of glycerol). The neotype is deposited in NTU; 3 specimens are deposited in SMNH; 7 specimens are deposited in TSUMZ.
Diagnosis. Body size: 315–398 × 199–249. Rostral, lamellar and interlamellar setae barbed, ro setiform, thinnest, le and in thickened, in usually thickest. Bothridial setae short, globular, barbed. Exobothridial setae well developed, setiform, barbed. Notogastral setae setiform, thin, smooth. Four pairs of saccules present. Subcapitular setae a shortest, ciliated, m (thinnest) and h (longest) barbed. Epimeral and anogenital setae setiform, thin, slightly barbed. Leg setae ft’ absent on tarsi I, solenidion φ on tibiae III and IV dilated distally.
Description. Measurements. Body length: 398 (neotype), 315–398 (other specimens); notogaster width: 249 (neotype), 199–249 (other specimens). Females larger than males: 381–398 × 232–249 versus 315–348 × 199–215.
Integument. Body color light yellow to light brown. Body surface and legs microfoveolate (visible under high magnification). Lateral parts of prodorsum between sublamellae and acetabula I, II microgranulate (diameter of granules less than 1).
Prodorsum (Figs 1a; 2a). Rostrum rounded. Lamellae located dorsolaterally, about half of prodorsum (measured in lateral view). Prolamellae present. Sublamellae thin, about 2/3 of lamellae. Sublamellar porose areas oval (6–8 × 4–6). Rostral setae (49–57) setiform, barbed unilaterally, lamellar (53–61) and interlamellar (65–73) setae thickened, slightly blunted distally, barbed; ro thinnest, in thickest (rarely le and in similar in thickness). Bothridial setae (20) globular, barbed, with short stalk and larger head. Exobothridial setae (28–36) setiform, thin, barbed. Dorsophragmata slightly elongated. Dorsosejugal porose areas elongate oval (12–16 × 4–6), transversely oriented, located posterolateral to interlamellar setae.
Notogaster (Figs 1a; 2a, 2b). Anterior notogastral margin truncate. Ten pairs of notogastral setae (14–16) setiform, thin, smooth. Four pairs of saccules with small openings and drop-like channels. Distance S1–S1 larger than S2–S2. Lyrifissures, opisthonotal gland openings, circumgastric scissure and circumgastric sigillar band distinct.
Gnathosoma (Figs 1c–1e). Subcapitulum longer than wide (77–82 × 61–65). Subcapitular setae setiform, a (16) shortly ciliated, m (20) and h (24) barbed; m thinnest. Two pairs of adoral setae (6–8) setiform, heavily barbed. Palps (length 53–57) with typical setation 0–2–1–3–9(+ω). Solenidion of palptarsi dilated mediodistally. Postpalpal setae (4) spiniform, smooth. Chelicerae (length 82–86) with two setiform, barbed setae, cha (32) longer than chb (20). Trägårdh’s organ of chelicerae elongate triangular.
Epimeral and lateral podosomal regions (Figs 1b; 2a). Humeral porose areas Ah elongate oval (12 × 6–8), Am absent. Epimeral setal formula: 3–1–3–3. Setae setiform, thin, slightly barbed; 1b, 3b, 3c, 4a, 4c (18–20) longer than other setae (14–16). Pedotecta I and II represented by small laminae, Pd II triangular, rounded in ventral view. Discidia slightly developed, rounded distally. Circumpedal carinae absent.
Anogenital region (Figs 1b; 2a, 2b). Four pairs of genital, one pair of aggenital, two pairs of anal and three pairs of adanal setae similar in length (14–16), setiform, thin, slightly barbed. Aggenital setae absent in two specimens. Adanal lyrifissures located close and parallel to anal plates. Adanal setae ad1 in posterolateral, ad2 and ad3 in lateral positions to anal aperture.
Legs (Figs 3a–3d). Median claws distinctly thicker than laterals, all barbed dorsally; lateral claws with tooth ventrodistally. Dorsoparaxial porose areas on femora I–IV and on trochanters III, IV and ventral porose areas in ventroposterior parts of tarsi and ventroanterior parts of tibiae well visible. Formulas of leg setation and solenidia: I (1–5–2–4–17) [1–2–2], II (1–5–2–4–15) [1–1–2], III (2–3–1–3–15) [1–1–0], IV (1–2–2–3–12) [0–1–0]; homology of setae and solenidia indicated in Table 1. Famulus of tarsi I short, erect, slightly dilated distally, inserted posterior to solenidion ω2. Setae ft’ absent on tarsi I. Solenidion φ on tibiae III and IV with drop-like dilatation distally.
Table 1.
Leg setation and solenidia of adult Dometorina taiwanica Tseng 1984
| Leg | Tr | Fe | Ge | Ti | Ta |
|---|---|---|---|---|---|
| I | v’ | d, (l), bv”, v” | (l), σ | (l), (v), φ1, φ2 | ft”, (tc), (it), (p), (u), (a), s, (pv), (pl), ɛ, ω1, ω2 |
| II | v’ | d, (l), bv”, v” | (l), σ | (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), ω1, ω2 |
| III | l’, v’ | d, l’, ev’ | l’, σ | l’, (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v’ | d, ev’ | d, l’ | l’, (v), φ | ft”, (tc), (p), (u), (a), s, (pv) |
Remarks. Dometorina taiwanica was described by Tseng (1984) from southern Taiwan, based on the holotype and 15 paratypes. Holotype and 4 paratypes: Kuanmaio, Tainan Hsien, leaf of Morus sp., 29.VI.1981 (Y.-H. Tseng); 4 paratypes: Fenchihu, Chiayi Hsien, Bangolia sp., 14.XII.1980 (Y.-H. Tseng); 1 paratype: Puli, Nantou Hsien, Polygonum hydropiper, 18.XI.1981 (S. C. Wu); 1 paratype: Tongyin, Chiayi Hsien, Cordia dichotoma, without data collection (Y.-H. Tseng); 5 paratypes: Tsushan, Nantou Hsien, Leleba sp., 8.VIII.1982 (Y.-H. Tseng). The neotype and 10 other specimens of D. taiwanica from the islands surrounding Taiwan (about 400 km from the type locality) are morphologically similar to the original description of the holotype and paratypes to the point where we did not observe any clear differences. However, we have noted the following nuance: Tseng (1984, p. 35–36) described the presence of eight pairs of notogastral setae and the absence of aggenital setae for this species. However, his Fig. 125 shows the development of ten pairs of notogastral setae, while our other specimens are with or without aggenital setae. Hence, D. taiwanica really has ten pairs notogastral setae. The presence and absence of aggenital setae is a morphological variability for this species.
(Figs 4–6)
Fig. 4.
Zygoribatula gratiosa Tseng 1984, adult: a – dorsal view (legs not shown); b – ventral view (gnathosoma and legs not shown); c – subcapitulum, ventral view; d – palp, right, antiaxial view; e – chelicera, left, paraxial view. Scale bar (µm): a, b – 50; c–e – 17.
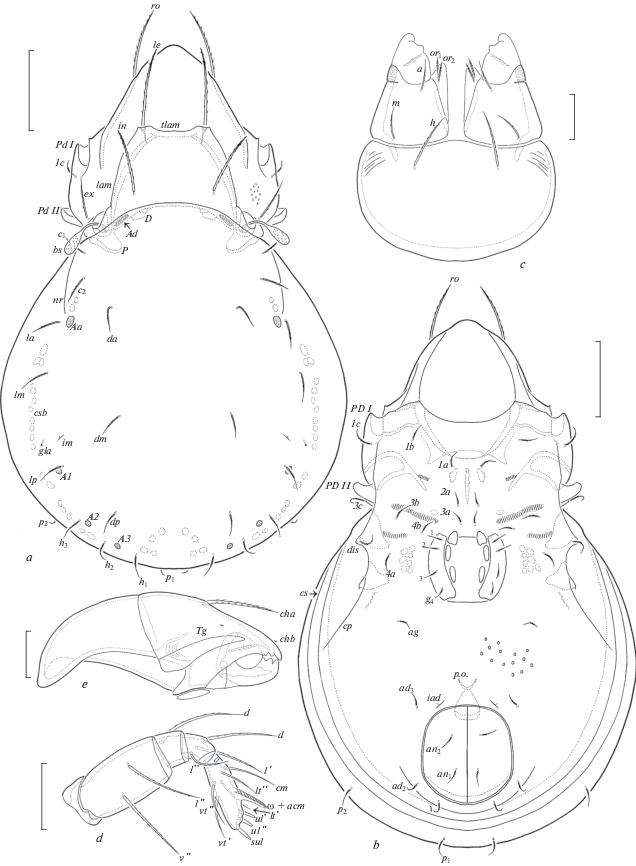
Fig. 5.
Zygoribatula gratiosa Tseng 1984, adult: a – lateral view (gnathosoma and legs not shown); b – posterior view. Scale bar 50 µm.
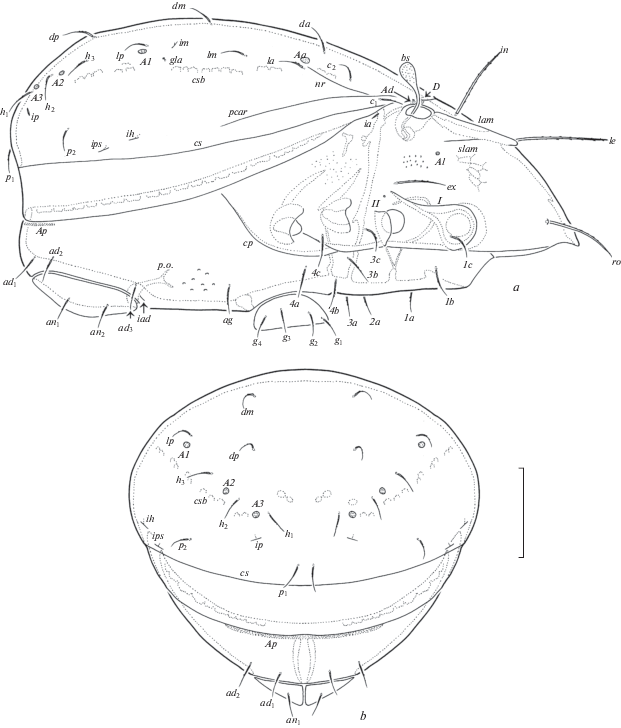
Fig. 6.
Zygoribatula gratiosa Tseng 1984, adult: a – leg I, right, antiaxial view; b – trochanter, femur, genu and basal part of tibia of leg II, ventral view; c – trochanter, femur and genu of leg III, left, antiaxial view; d – leg IV, left, antiaxial view. Scale bar 20 µm.
Material examined. Neotype (♀) and 9 specimens (6 ♀♀, 3 ♂♂): locality #7, Taiwan, New Taipei City, Wulai, Drulu, 24°49.544′ N, 121°30.437′ E, 884 m a. s. l., soil, 13.VII.2017 (J.-R. Liao, H.C. Lee).
Type deposition (all in ethanol with drop of glycerol). The neotype is deposited in NTU; 3 specimens are deposited in SMNH; 6 specimens are deposited in TSUMZ.
Diagnosis. Body size: 298–365 × 182–232. Rostral, lamellar and interlamellar setae setiform, barbed, le longest, ro often thinnest. Lamellar cusps truncate or with indistinct lateral and medial teeth. Translamella thick, straight. Bothridial setae short, clavate, barbed. Exobothridial setae well developed, setiform, barbed. Anterior notogastral margin with one pair of longitudinal humeral ridges directed to porose areas Aa or setal alveoli la. Thirteen pairs of notogastral setae short, setiform, slightly barbed. Four pairs of porose areas small, rounded. Subcapitular setae setiform, barbed, h longer and thicker than a and m. Epimeral and anogenital setae setiform, slightly barbed. Ventral region between genital and anal apertures foveolate.
Description. Measurements. Body length: 332 (neotype), 298–365 (other specimens); notogaster width: 215 (neotype), 182–232 (other specimens). Females larger than males: 332–365 × 199–232 versus 298–315 × 182.
Integument (Figs 4a–4c). Body color light brown. Body surface and legs microfoveolate (visible under high magnification). Lateral parts of prodorsum between sublamellae and acetabula I, II microgranulate (diameter of granules less than 1) and tuberculate (diameter of tubercles up to 1). Anterolateral part of subcapitular mentum striate. Ventral region between genital and anal apertures sparsely foveolate (diameter of foveoles up to 2).
Prodorsum (Figs 4a; 5a). Rostrum indistinctly protruding, rounded. Lamellae located dorsolaterally, shorter than half of prodorsum (measured in lateral view). Lamellar cusps truncate, sometimes with indistinct lateral and medial teeth. Translamella thick, straight. Prolamellae absent. Sublamellae thin, about 1/2 of lamellae. Sublamellar porose areas rounded (2–6) or oval (4–6 × 2–4). Rostral setae (45–49) setiform, barbed unilaterally, lamellar (53–57) and interlamellar (45–49) setae setiform, erect, slightly blunted distally, barbed; ro often thinner than le and in. Bothridial setae short (32–36), clavate, barbed, stalk and head similar in length. Exobothridial setae (32–36) setiform, thin, barbed. Dorsophragmata semi-oval. Dorsosejugal porose areas elongate oval (12–16 × 4–6), transversely oriented, located posterolateral to interlamellar setae.
Notogaster (Figs 4a; 5a, 5b). Anterior notogastral margin convex, with one pair of longitudinal humeral ridges directed to porose areas Aa or setal alveoli la. Pleural carinae well developed. Thirteen pairs of notogastral setae (16–20) setiform, thin, slightly barbed. Four pairs of porose areas rounded, Aa (10–12) usually larger than A1, A2, A3 (8–10). Distance A1–A1 larger than A2–A2. Lyrifissures, opisthonotal gland openings, circumgastric scissure and circumgastric sigillar band distinct.
Gnathosoma (Figs 4c–4e). Subcapitulum longer than wide (82–86 × 65–69). Subcapitular setae setiform, barbed, h (20–24) longer and thicker than a (12–16) and m (12–16). Two pairs of adoral setae (6–8) setiform, heavily barbed. Palps (length 53–57) with typical setation 0–2–1–3–9(+ω). Solenidion of palptarsi bacilliform. Postpalpal setae (4) spiniform, smooth. Chelicerae (length 82–90) with two setiform, barbed setae, cha (24–28) longer than chb (16–18). Trägårdh’s organ of chelicerae elongate triangular.
Epimeral and lateral podosomal regions (Figs 4b; 5a). Humeral porose areas absent. Epimeral setal formula: 3–1–3–3. Setae (16–20) setiform, thin, slightly barbed. Pedotecta I and II represented by small laminae, Pd II trapezoid, rounded in ventral view. Discidia triangular, rounded distally. Circumpedal carinae short, directed to acetabula IV.
Anogenital region (Figs 4b; 5a, 5b). Four pairs of genital, one pair of aggenital, two pairs of anal and three pairs of adanal setae similar in length (16–20), setiform, thin, slightly barbed. Adanal lyrifissures located close and anterior to anal plates. Adanal setae ad1 in posterior, ad2 in posterolateral, ad3 in anterolateral positions to anal aperture. Postanal porose areas band-like (49–57 × 4–6).
Legs (Figs 6a–6d). Median claws distinctly thicker than laterals, all barbed dorsally; lateral claws with tooth ventrodistally. Tibiae I, II with small triangular process. Dorsal porose areas on tarsi I, dorsoparaxial porose areas on femora I–IV and on trochanters III, IV and ventral porose areas in ventroposterior parts of tarsi and ventroanterior parts of tibiae well visible. Formulas of leg setation and solenidia: I (1–5–3–4–20) [1–2–2], II (1–5–2–4–16) [1–1–2], III (2–3–1–3–15) [1–1–0], IV (1–2–2–3–12) [0–1–0]; homology of setae and solenidia indicated in Table 2. Famulus of tarsi I short, erect, slightly dilated distally, inserted posterolateral to solenidion ω2.
Table 2.
Leg setation and solenidia of adult Zygoribatula gratiosa Tseng 1984
| Leg | Tr | Fe | Ge | Ti | Ta |
|---|---|---|---|---|---|
| I | v’ | d, (l), bv”, v” | (l), v’, σ | (l), (v), φ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), (pl), v’, l”, ɛ, ω1, ω2 |
| II | v’ | d, (l), bv”, v” | (l), σ | (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), l”, ω1, ω2 |
| III | l’, v’ | d, l’, ev’ | l’, σ | l’, (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v’ | d, ev’ | d, l’ | l’, (v), φ | ft”, (tc), (p), (u), (a), s, (pv) |
See Table 1 for explanations.
R e m a r k s. Zygoribatula gratiosa was described by Tseng (1984) from central (holotype) and eastern (paratypes) Taiwan, based on the holotype and 2 paratypes. Holotype: Lushan, Nantou Hsien, litter of maple leaf, 16.I.1982 (Y.-H. Tseng); 2 paratypes: Nanshan, Yilan Hsien, lichen, 21.VIII.1981 (L. L. Lai). The neotype and other specimens of Z. gratiosa from northern Taiwan (about 100 km from the type locality) are morphologically similar to the original description of the holotype and paratypes to the point where we did not observe any clear differences. However, we have noted the following nuance: Tseng (1984, p. 27) described the presence of 12 pairs of notogastral setae. However, we think that this statement is wrong because his figures (95, 98) do not show the places of insertions of notogastral setae p2, which, perhaps, have neither been noted nor counted. Most likely, p2 is present and in reality, Tseng’s specimens had 13 pairs of notogastral setae (including p2), similarly to our specimens.
Список литературы
Ermilov S.G., Liao J.-R., 2017. Contribution to the knowledge of the oribatid mite genus Brassiella (Acari: Oribatida: Caloppiidae) // Biologia. V. 72. № 9. P. 1041–1048.
Ermilov S.G., Liao J.-R., 2017a. Neotype designations and supplementary descriptions of Taiwanoppia subtropica Tseng, 1982 and Muliercula chiayiensis Tseng, 1984 (Acari: Oribatida: Oppiidae, Scheloribatidae) // Systematic and Applied Acarology. V. 22. № 7. P. 897–914.
Ermilov S.G., Liao J.-R., 2017b. New faunistic and taxonomic data on oribatid mites (Acari, Oribatida) of Taiwan // Systematic and Applied Acarology. V. 22. № 6. P. 824–840.
International Commission on Zoological Nomenclature, 1999. International code of zoological nomenclature, fourth edition. London: International Trust for Zoological Nomenclature. 306 p.
Liao J.R., Ho C.C., Ko C.C., 2017. Amblyseius bellatulus Tseng (Acari: Phytoseiidae): neotype designation with first description of a male // Acarologia. V. 57. № 2. P. 323–335.
Norton R.A., 1977. A review of F. Grandjean’s system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae // Dindal D.L., ed. Biology of oribatid mites. Syracuse: SUNY College of Environmental Science and Forestry. P. 33–61.
Norton R.A., Behan-Pelletier V.M., 2009. Oribatida // A Manual of Acarology (TX). Lubbock: Texas Tech University Press. P. 430–564.
Tseng Y., 1982. Taxonomical study of oribatid mites from Taiwan (Acarina: Astigmata) (I) // Chinese Journal of Entomology. V. 2. № 1. P. 53–106.
Tseng Y., 1984. Taxonomical study of oribatid mites from Taiwan (Acarina: Astigmata) (II) // Chinese Journal of Entomology. V. 4. P. 27–74.
Travé J., Vachon M., 1975. François Grandjean. 1882–1975 (Notice biographique et bibliographique) // Acarologia. V. 17. № 1. P. 1–19.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


