Зоологический журнал, 2020, T. 99, № 12, стр. 1363-1374
Impact of Zebra Mussel Dreissena polymorpha Pallas 1771 (Bivalvia) Appearance on fish populations in Lake Pleshcheevo, European Russia
A. K. Smirnov a, *, D. D. Pavlov a, **, Yu. V. Kodukhova a, ***, D. P. Karabanov a, ****
a Papanin Institute for the Biology of Inland Waters, Russian Academy of Sciences
152742 Yaroslavl Region, Borok, Russia
* E-mail: smirnov@ibiw.ru
** E-mail: tukki@bk.ru
*** E-mail: jukod@ibiw.ru
**** E-mail: dk@ibiw.ru
Поступила в редакцию 25.04.2020
После доработки 27.04.2020
Принята к публикации 30.04.2020
Аннотация
The study analyzes structural changes in the ichthyofauna of Lake Pleshcheevo following the introduction of Dreissena polymorpha (Pallas 1771) (Bivalvia). Trends in the functioning of the community were evaluated based on a comparison of the literature (prior to the introduction) and our observations (after the formation of a sustainable zebra mussel biocenosis). All fish species remained in the lake, but catches changed significantly. Gillnets set in the littoral and sublittoral zones during the feeding period consist mainly of large roach and perch while vendace were prevalent in the pelagic zone. The abundance of benthophagous fishes increased slightly due to the presence of the zebra mussel which made up a significant part of the diet followed by an increase (p < 0.05) in the growth rates of roach and silver bream. The growth rates of the bream, which prefers soft zoobenthos, decreased. In addition, the formation of a stable biocenosis of zebra mussel in the lake probably caused changes in the spatial structure of the fish community. What is notable is the disappearance of small roach from pelagic assemblages, probably due to alterations in the trophic links of the littoral and sublittoral zones, as well as the expected increase in food competition among pelagic fish species.
Aquatic ecosystems of Central Russia have been subject to anthropogenic pressure following the creation of recreational sites and major infrastructure projects during the 20th century. The relevance of the study of such ecosystems is enshrined in Agenda 21 of the Rio-de-Janeiro United Nations Declaration on Environment and Development (UNDED, 2012), where Chapter 15 “Conservation of biological diversity” and Chapter 18 “Protection of the quality and supply of freshwater resources…” are highlighted separately. It also takes account of an inventory and conservation of local and relict forms when assessing the biological diversity of aquatic ecosystems. One such water body is Lake Pleshcheevo, located in the south-west of the Yaroslavl region, Russia. The relict population of the European vendace, Coregonus albula (Linnaeus 1758) inhabiting this lake, is included in the Red Book of the Russian Federation (Reshetnikov, 2010). The Lake Pleshcheevo National Park was founded in 1988 in order to protect this relict population and the overall ecosystem of the lake and surrounding area. Environmental protection and monitoring carried out over a forty-year period has enabled the evaluation of changes that have taken place.
The introduction of alien organisms into new habitats can entail both predictable as well as some unknown impacts (Nalepa, Schloesser, 2013; Jernelöv, 2017; Dgebuadze et al., 2018). Increases in the populations of dreissenids, firstly Dreissena polymorpha (Pallas 1771) (Mollusca, Bivalvia), took place in the 1960s and 70s in many reservoirs of the Upper Volga resulting in impacts on aquatic ecosystems. There have been changes in water quality, habitats for many species of invertebrates and fish. These changes have influenced the state of fish populations and changes in trophic links in ecosystems (Nalepa, Schloesser, 2013; Lazareva, 2018).
Zebra mussel has appeared in Lake Pleshcheevo later, in the 1980s, having successfully occupied a niche on the bottom ecosystem (Shcherbina, 2008) over several years. D. polymorpha maintained a high level of abundance and druses and shells accumulated to form extensive deposits at depths of 4.5–12 m. The increased levels of filtration contributed to changes in the trophic structure of the lake (Shcherbina, 2009; Lazareva, 2018).
Several changes to the ecosystem of Lake Pleshcheevo followed the introduction of the zebra mussel (Stolbunova, 2006; Zhdanova, 2018; Zhdanova et al., 2019; Pryanichnikova, Tsvetkov, 2018; Pryanichnikova, 2019). However, only two fish species have been studied, the roach (Kasyanov, Izyumov, 1995; Shcherbina, 2008; Stolbunov, 2008; Kodukhova, Karabanov, 2017) and the vendace (Borovikova, 2017; Borovikova, Artamonova, 2018). Changes that have taken place in the Rybinsk Reservoir have shown that the zebra mussel can cause rearrangement of the trophic links and the spatial distribution of fishes (Gerasimov, 2015). This study examines structural changes in the ichthyofauna of Lake Pleshcheevo, following the introduction of the zebra mussel.
MATERIALS AND METHODS
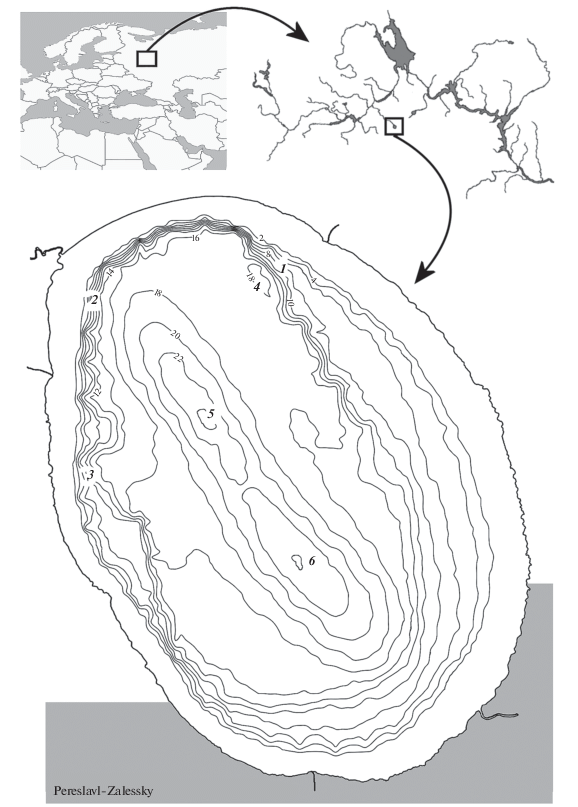
Lake Pleshcheevo (56° N, 38° E) is located in Central Russia and is an oval water body of glacial origin. The coastline is 27 km, area – 51.5 km2. The littoral zone with depths of up to 3 m makes up about 20% of the lake area. The greatest depths (max 24 m) are noted in the northwestern part of the lake. There is an urban agglomeration – the City of Pereslavl-Zalessky on the south coast. The lake belongs to the Upper Volga basin and intercommunicates with it through the only outflowing river Veksa (Fig. 1). A dump dam was built on the river 5 km from its source to regulate the flood level, which provides significant isolation of the lake from the Volga basin. A layer of dead shells of mollusks is present from 2 m depth and deeper, reaching the highest density in the sublittoral zone at 4–12 m. With an increase in depth of more than 14 m, the gravitational displacement of finely dispersed fractions leads to the formation of a thick surface layer of olive and black (at depths of more than 18 m) silts that impede the life of the zebra mussel. According to the hydrochemical composition of the water, the lake is a freshwater medium-saline water body. Electrical conductivity of water during the study period varied within rather narrow boundaries – from 275 to 300 μS/cm. Secchi disk depth is quite high, ranging from 2 to 6 m, depending on the season of observations. Sunlight penetrates to a depth of 6 m with a noticeable decrease in illumination to 50–100 lux at a horizon of 13–15 m. The boundary of the lower limit of photosynthesis (0.1% of incoming radiation) is currently located at depths of 13–16 m. In general, Plescheevo Lake can be described as a water body with high water quality, and due to the poor development of coastline geomorphology (the absence of large bays, swampy floodplains, large tributaries), the entire littoral of the lake provides fairly equal conditions for the habitat of various fish species.
Fig. 1.
The geographical position of Lake Pleshcheevo in the Upper Volga basin and the location of sites where gill nets were set in the littoral and sublittoral zones – 1, 2, 3, as well as its pelagic zone – 4, 5, 6.
The lake is a typical dimictic water body with water homothermia in spring and autumn, and pronounced temperature stratification of the water column in summer and winter (Butorin, Sklyarenko, 1989). In the period of spring fishing (early May), the temperature of the surface water layer (0–2 m) was 7–10°С, briefly warming up to 17°С in the daytime. At this time of the year heat transfer to the bottom layers of the water occurs only due to diffusion and wind mixing, therefore at depths of over 6 m the temperature remained at 4°С. Further warming up (early June) led to the formation of a clearly identifiable thermocline in the lake at 6–8 m horizons. Surface water temperature could reach >20°С, being 10°С in the metalimnion and close to 5°С in the hypolimnion. In the autumn period of fishing (mid-September–October), the destruction of thermocline structures was observed and, as a result, the entire water column was cooled to 10°C.
Provision with oxygen, one of the key factors for the life of hydrobionts, has changed in close connection with temperature. Over the entire observation period, there was no significant deficit at all coastal stations. In spring (May), the concentration of oxygen dissolved in water in the littoral of the lake was 11–14 mg/L. Summer heating of lake’s water led to the appearance of pronounced oxycline, however the dissolved oxygen content was 5–6 mg/L even at 12–18 m depths. A critical level of oxygen deficiency (about 1 mg/L) was noted only in the summer period for deep (more than 18 m) zones of the central part of the lake. With autumn (mid-September–October) destruction of the thermocline, the oxycline also disappeared, and the dissolved oxygen concentration was the same (12–13 mg/L) throughout the entire water column.
Fish were sampled in the field seasons 2014–16 from April to October. The total exposure of gill nets was 760 h. The main fishing gears were set gill nets: mesh 45 mm, size 2 × 70 m – 1 pc.; mesh 55 mm, size 1.8 × 35 m – 1 pc.; fragmented mesh net 10–50 mm (×10) size 1.8 × 30 m – 14 pcs.; fragmented mesh net 10–50 mm (×10) size 0.9 × 30 m – 3 pcs. Fishing was carried out in three areas (Fig. 1): in the area of the village of Solomidino (1), near Urev settlement (2) and near the mouth of the river Kukhmarka (3). The first two biotopes are a typical littoral with a smooth lowering of the bottom, and the last is the estuary site. The nets were set on isobaths every 2 m, starting from 1 m depth and down to 12 m. Pelagic 6 × 30 m nets with 10–24 mm mesh set in the surface and bottom layers of water (Fig. 1, stations 4, 5, 6) were used in the open part of the lake with depths of over 18 m. Young fish (predominantly 0+) were caught in the coastal area using a fry drag (length 9 m, height 1.5 m, mesh in the wings and patch 4 mm), fry seine (length 24 m, mesh in the wings 12 mm, mesh in the patch 9 mm) and an ichthyological lift (size 1.5 × 1.5 m, mesh 3 mm) and an ichthyological net 0.4 m in diameter with a mesh of 2 mm.
The entire catch was processed according to the standard method (Pravdin, 1966) with species identification and determination of the main biological characteristics of fish. Morphological analysis was performed utilizing quantitative (meristic), plastic and alternative nonmetric (pharyngeal teeth formula) features. The patterns of individual fish growth were investigated using various registering structures – cleithrum, operculum and scales. Linear growth was analyzed according to direct individual determination of fish body length and age using the generally accepted methods (Chugunova, 1963). The work involved extensive archival material of the laboratory of fish ecology of the IBIW RAS on the size-age series and morphological data of the osteological collection of the IBIW RAS. In total, to study the size and age characteristics of cyprinid fish populations, the following were analyzed: 375 individuals of roach from Lake Pleshcheevo from 1979–1981, 513 from 2014–2016 and 491 from the Rybinsk reservoir from 2010–2015; 188 individuals of silver bream from the Lake Pleshcheevo from 1979–1981, 399 from 2014–2016 and 474 from the Rybinsk reservoir from 2010–2015; 212 bream individuals from Lake Pleshcheevo 1979–1981, 479 for 2014–1916 and 511 from the Rybinsk reservoir from 2010–2015.
The significance of differences in growth dynamics was assessed using the non-parametric chi-square test (Ivanter, Korosov, 2003). Statistical processing of the material was carried using Statistica v.6.1 software package (StatSoft, Inc., USA).
RESULTS
The fish species composition in Lake Pleshcheevo has not changed much over the past 40 years. During the period of studies, we found 13 native fish species. Cyprinids (Cyprinidae) were the most represented group in the fish community of the lake: roach Rutilus rutilus (Linnaeus 1758), bleak Alburnus alburnus (Linnaeus 1758), bream Abramis brama (Linnaeus 1758), silver bream Blicca bjoerkna (Linnaeus 1758), ide Leuciscus idus (Linnaeus 1758), tench Tinca tinca (Linnaeus 1758) crucian carp Carassius carassius (Linnaeus 1758) and gibel carp Carassius auratus complex. In addition, gill net catches also contained representatives of coregonids (Coregonidae) – European vendace Coregonus albula (Linnaeus 1758); gadids (Gadidae) – burbot Lota lota (Linnaeus 1758); esocids (Esocidae) – European pike Esox lucius Linnaeus 1758; percids (Percidae) – Eurasian perch Perca fluviatilis Linnaeus 1758 and common ruffe Gymnocephalus cernuus (Linnaeus 1758); cobitids (Cobitidae) – spined loach Cobitis taenia Linnaeus 1758. An invasive species, Amur sleeper Perccottus glenii (Dybowski 1877) (fam. Odontobutidae) was found in coastal water bodies (ponds in the city of Pereslavl-Zalessky).
Littoral zone (depths 0–6 m). In catches carried out by the fry drag, ichthyological lift and ichthyological net in the coast of the lake, yearlings of roach (50–60%) and perch (28–32%) prevailed, while the proportion of bleak was relatively small (7–10%). Juvenile bream (0.2–5%), ide (1–3%), and silver bream (~1%) can be distinguished among other species. Single yearlings of the remaining species (Carassius sp., T. tinca, C. taenia, L. lota and E. lucius) were caught.
Fragmented gill nets set at depths from 1 to 6 m, performed in 2014–2016 revealed dominance in the catches of the coast of different-sized individuals of roach and perch (Fig. 2). During the whole feeding season, these two species together accounted for about 80% of the number of fish caught, with a certain predominance of roach (64–71%). Bream, silver bream and bleak individuals were caught significantly less frequently (from 3 to 11% depending on the season) (Fig. 2). As a rule, small-sized pike (SL ≤ 500 mm), ruffe and ide were present in small amounts (not more than 5%) in catches of the coastal zone, and even less often, tench and burbot.
Fig. 2.
The species composition of gill net catches in different ecological zones of Lake Pleshcheevo at different periods of feeding: a – May–June, b – September–October.
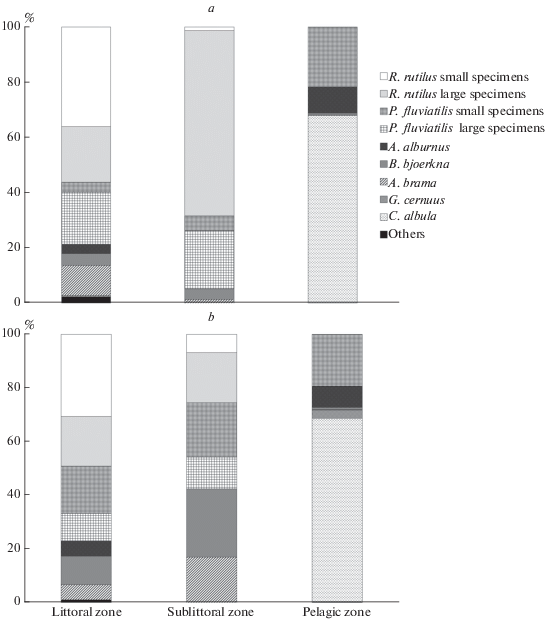
Sublittoral transitional zone (depth 6–12 m). Large individuals of roach and perch prevailed (up to 90% of the catch) in the catches of bottom gill nets at the beginning of the feeding period (Fig. 2a). The proportion of small specimens was relatively low – 1 and 5%, respectively. The number of caught bream and silver bream was also small and rarely exceeded 5%. During autumn, the dominance of roach and perch generally remained (~60%), however, the proportion of small individuals among them increased significantly (Fig . 2b). At the end of the feeding season, bream and silver bream were caught in the sublittoral in substantially large quantities (17 and 25%, respectively). Other fish were virtually absent in net catches in this area of the lake.
Pelagic zone. The set pelagic nets revealed dominance of vendace (up to 70% of the number of fish caught) in the pelagic zone. The occurrence of other species in the water column was significantly lower (Fig. 2). So, the second largest number in the catches was taken by small individuals of perch (about 20%), bleak was recorded even less frequently (up to 10%). In addition, there were few (≤5%) individuals of silver bream, ruffe, large perch, small bream and pike in the pelagic zone. The virtually complete absence of roach in the pelagic zone is noteworthy.
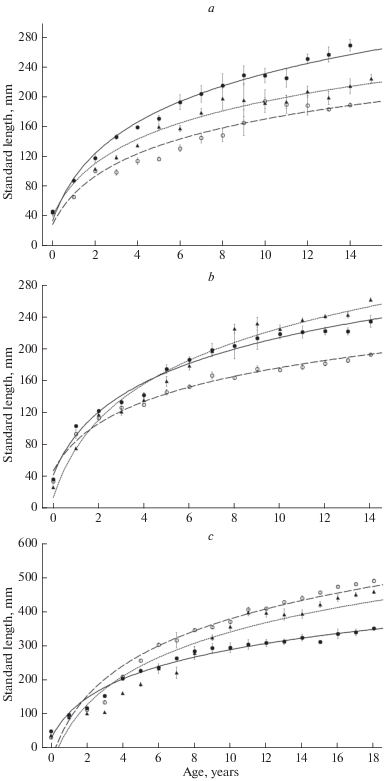
The appearance and successful acclimatization of zebra mussel in Lake Pleshcheevo caused certain changes in the population characteristics of individual fish species. The inclusion of the mollusk into the diet of roach has significantly (p <0.05) improved the linear-age indices of individuals (Fig. 3a). The growth rate of the silver bream, also actively consuming mollusks, significantly increased (p < 0.05) in a similar way (Fig. 3b). At the same time, we noted a deterioration (p < 0.05) in linear-age indices of another benthophage species – bream, which occurred after the introduction of D. polymorpha (Fig. 3c).
Fig. 3.
The growth rate of some cyprinid species (a – roach, b – silver bream, c – bream) in Lake Plescheyevo before (- - -) and after (—) the introduction of zebra mussel; data from the Rybinsk Reservoir (…) for 2010–2015 are presented for comparison. Mean values ± 95% confidence interval are presented.
DISCUSSION
The fish species composition in Lake Pleshcheevo has not changed much over the past 40 years according to our own and literature data (Malinin, Linnik, 1983; Butorin, Sklyarenko, 1989). Of the typical species noted earlier (Strelnikov, Permitin, 1983), we did not meet only the sunbleak Leucaspius delineatus (Heckel 1843) and the gudgeon Gobio gobio (Linnaeus 1758), which may be explained by the limited material from small rivers – tributaries of the lake. In addition, no invasive fish species were found in the lake, most likely due to its isolation from the Volga’s basin. The Amur sleeper found in coastal ponds, was not found in the lake itself, probably due to its increased vulnerability to large piscivorous predators, pike in particular (Smirnov et al., 2019).
Littoral zone (depths 0–6 m). Fry of various fish species actively utilize shallow parts of lakes usually overgrown with aquatic vegetation as natural refugia and feeding sites (Eklov, 1997; Smokorowski, Pratt, 2007; Gerasimov, 2015). At the same time, adult individuals of some peaceful and predatory fish occur here on a permanent basis or temporarily (horizontal feeding migrations). Juveniles of bleak (on average 50–80%), perch (up to 46%) and roach (30–50%) constituted the major part of minnow seine catches in the early 80s in the Lake Pleshcheevo littoral. Juveniles of bream and silver bream (less than 10%), ruffe (less than 1%), pike (less than 1%) and some other species were recorded in much smaller quantities (Malinin, Linnik, 1983; Butorin, Sklyarenko, 1989). Comparing these and own data we can note that at present the proportion of bleak individuals of the younger age classes (0+) has decreased by more than 5 times. At the same time, in littoral catches the share of roach fingerlings has increased slightly and is currently more than 50%. It is worth noting that for all three years of observations, when analyzing more than five thousand immature fish, only one hybrid individual was found. In this respect, Lake Pleshcheevo differs markedly from the Rybinsk reservoir, where in low-level years, supplemented by unfavorable temperature conditions, up to 1.5% of hybrids may appear among young carp fish (Kodukhova, 2011). Apparently, we can point out the greater level regime stability of Lake Pleshcheevo in comparison with the reservoirs of the Volga river as a factor determining such differences.
Before analyzing the species composition of net catches, it should be noted that roach and perch are represented in the lake by two ecological groups, noted as far back as the 1970s and 1980s and preserved to the present (Malinin, Linnik, 1983; Shcherbina, 2008; Kodukhova, Karabanov, 2017). One of these groups, more numerous, is formed by individuals of a younger age and smaller size, previously included in vast pelagic aggregations and mainly feeding on zooplankton. The other is represented by individuals of different ages, constantly living in the littoral and sublittoral zones of the lake and consuming benthic and epibenthic organisms, as well as juvenile fish (large perch). Given this fact, as well as for a more complete analysis of the obtained material, we divided the totality of all caught specimens of roach and perch into two size groups. Individuals with a body length of less than 150 mm (except for the age class 0+) were assigned to “small” fish, the rest were included in the group of “large” ones. Previously, such a division was used by some researchers to isolate intraspecific forms of roach from various water bodies (Kasyanov et al., 1981; Kodukhova, Karabanov, 2017).
Bleak, large roach and large perch constituted the base of gill net and seine catches in the littoral zone of the lake in the early 1980s (Malinin, Linnik, 1983; Butorin, Sklyarenko, 1989). Silver bream, ruffe, small-sized individuals of bream, as well as small and medium pike (mainly in overgrown coastal areas) were found here in lesser numbers. Roach and perch of different sizes are currently dominant in catches in the littoral (Fig. 2). Bleak that has been abundant in the past has become noticeably less frequent (3–6%) in the gill net catches. At the same time, ide is more often seen in the catches nowadays. When comparing literature and modern data, it becomes evident that changes in the lake’s ecosystem over the past decades have mainly affected the ratio of the number of species in littoral aggregations, with their species composition remaining unchanged.
Sublittoral transitional zone (depths 6–12 m). Areas of relatively sharp changes in depths – slopes, are considered one of the most productive zones of lakes and reservoirs (Smokorowski, Pratt 2007). These areas typically serve as the main biotopes for benthophagous fish (Gerasimov, 2015). This ecological group accounted for no more than 14% of the total fish biomass in Lake Plescheevo according to studies of the late 1970s and early 1980s (Malinin, Linnik, 1983; Butorin, Sklyarenko, 1989). At the same time, the species ratio was quite even and was determined by approximately equal shares of bream, ruffe, roach and silver bream. With the exception of large roach, which was more common in the littoral zone during the feeding season, mature individuals of all the aforementioned species were distributed in a relatively narrow strip of depths from 5 to 12 m along the entire perimeter of the lake, usually not forming dense aggregations. Large specimens of perch and pike stayed in this zone as well. It is noteworthy that the most numerous pelagic aggregations of bleak, roach and perch were also confined to the water column above the sublittoral transition zone (depths 5–16 m). The data presented above demonstrate significant differences in the catches of fish in the sublittoral zone that occurred after the introduction of zebra mussel into the lake (Fig. 2). Thus, the proportion of large individuals of roach as well as the number of caught silver bream (especially at the beginning of the feeding season) has currently increased.
Pelagic zone. A significant predominance of pelagic complex fish was observed in Lake Plescheevo at the beginning of the 80s. This complex mainly consisted of bleak, small individuals of roach, perch and vendace (Malinin, Linnik, 1983; Butorin, Sklyarenko, 1989). Inhabiting the pelagic zone, these species formed aggregations with pronounced spatial disunity, which was most pronounced during the lake’s summer stratification. The schools of bleak, occupying the upper layers of the epilimnion (0–5 m from the surface), were found in virtually the entire area of the lake with an average density 0.25 ind./m2. Small individuals of roach and perch inhabited the middle layer of the epilimnion (5–8 m from the surface), mainly concentrating above 5–13 m isobaths. Vendace shoals were more often recorded in the central part of the lake (about 30% of the area) closer to the border with metalimnion (8–12 m from the surface) with an average density of 0.02 ind./m2. Ruffe, silver bream and predators (pike, burbot, large perch) were caught in the open part of the lake in small numbers in addition to the noted species. To date, the pelagic part of lake’s fish community has apparently undergone significant changes (Fig. 2). First of all, attention is drawn to the fact of overwhelming dominance of vendace in the catches. As in the past individuals of this species often occupy the lower layers of the epilimnion in the Central part of the lake during the period of intensive feeding, that is determined by a combination of abiotic and biotic factors, and primarily by the temperature and content of dissolved oxygen (Butorin, Sklyarenko, 1989; Gerasimov et al., 2019). At present, the share of small perch in catches has significantly decreased, which in the past formed up to 50–75% of the composition of pelagic aggregations. Also, previously numerous individuals of bleak and small roach lost their positions while the latter actually ceased to be registered in pelagic nets.
The presence of an abundant pelagic fish complex in Lake Plescheevo in 1970–80s was explained by the high biomass of zooplankton, developing due to the significant eutrophication of this water body due to anthropogenic impact (Butorin, Sklyarenko, 1989; Stolbunova, 2006). At the same time, researchers of that time noted relatively low growth rates of pelagic species owing to their high abundance and intraspecific competition (bleak), as well as to the feeding with uncharacteristic food (roach, perch and silver bream). The lake population of vendace was depressed at that time (Butorin, Sklyarenko, 1989).
Probably, an equally important factor determining the predominance of the pelagic complex of fish in the lake was the absence of predators specific to this zone, in particular zander Sander lucioperca (Linnaeus 1758). Small pikes and large perches found in the water column have only partially occupied this ecological niche (Butorin, Sklyarenko, 1989). The significance of this biotic factor for the existence of pelagic assemblages of peaceful fish, may be proven on the example of zander introduction into one of the lakes in Norway (Braband, Faafeng, 1993). The introduction of such a predator to the ecosystem caused a sharp decrease in the density of pelagic assemblages of immature roach from 12 000–15 000 to 250 ind./ha. No less interesting are the experiments of Persson (Persson, 1993), in which fry dramatically changed the feeding spectrum from zooplankton to detritus-algae in the presence of predators. There are other examples of a similar effect of pelagic predators on the assemblages of small individuals of different species of fish living in the water column in the literature (Smokorowski, Pratt, 2007).
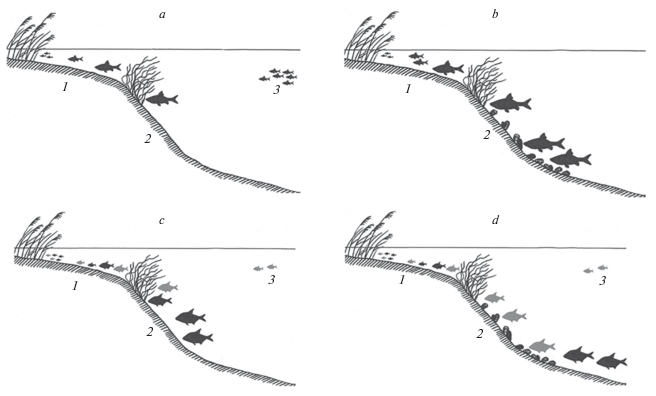
Along with this, in a specialized monograph (Butorin, Sklyarenko, 1989), devoted to the general characteristics of the lake ecosystem, noted the presence of biotic and abiotic factors suppressing the increase in the number of benthophagous fish. Thus, the lack of suitable spawning grounds was decisive for the bream population, severe helminth infestation (ligulosis) for the silver bream, summer killing phenomena occurring in the bottom layers of water for the ruffe, and the specific composition of benthic food organisms for roach. At the same time, good conditions for feeding and a low number of benthivorous fish ensured their relatively high growth rates (Butorin, Sklyarenko, 1989). Nevertheless, despite the significant total benthos biomass (Bakanov, 1983), the dominance of oligochaetes and chironomids in its composition with a pronounced decrease in the proportion of mollusks negatively affected the abundance and growth rates of some species, especially roach. It is likely that such specific benthic composition, against the background of high zooplankton biomass and the absence of specialized pelagic predators, caused the spatial redistribution of a significant part of the roach population to the lake pelagial, with the formation of large assemblage of small individuals (Fig. 4a).
Fig. 4.
Changes in the spatial distribution of some fish species in the littoral (1), sublittoral (2) and pelagic (3) zones of Lake Plescheyevo after the introduction of zebra mussel: a – roach in 1978–1981, b – the same in 2014–2016, c – bream (black) and silver bream (gray) in 1978–1981, d – the same in 2014–2015.
Significant changes in the lake’s ecosystem have occurred only in the late 1980s after an unintentional introduction of zebra mussel and its successful acclimatization. After a short period, this mollusk species became the main food component in the diet of large roach (Scherbina, 2008). Due to the high nutritional value of mollusk tissues (Mackie, Schloesser, 1996), this had a positive effect on the growth rates of roach individuals, with modern indicators of which significantly exceed not only those previously noted for the lake population, but also for the roach of the Rybinsk Reservoir (Fig. 3a). In addition, an increase was recorded in the maximum fish size (>300 mm versus 215–220 mm in the past), as well as their life expectancy. It was previously shown that the formation of a “mollusk-eating” roach morphotype began since the penetration of zebra mussel into Lake Pleshcheevo (Kodukhova, Karabanov, 2017). The emergence of a similar morphotype, characterized by massive pharyngeal teeth, a high rate of linear and weight growth, was also noted for other water bodies colonized by D. polymorpha (Gerasimov, 2015). In addition, it should be noted that the increase in the food value of macrozoobenthos after the introduction of zebra mussel occurs not only directly due to it itself, but also due to an increase in the number and species richness of bottom invertebrates associated with zebra mussel (Scherbina, 2008; Jernelöv, 2017).
However, at present, roach has virtually ceased to be found in the pelagic zone of Lake Pleshcheevo (Fig. 4b). The main reason is the changing conditions for feeding of individuals of this species in various ecological zones. The decrease of trophic status of the lake, as a result of mollusk’s filtration activity, has apparently exacerbated food competition among pelagic species and contributed to the spatial redistribution of roach aggregations. Studies of North American scientists performed in Lake Erie have shown that the introduction of zebra mussel caused a decrease in the density of phytoplankton accumulations by 90% and an abundance of zooplankton by 55–71% (Jernelöv, 2017). This, of course, entails a significant revision of the competitive relationships of fish, the basis of which are planktonic organisms.
Mollusks, and, in particular, zebra mussel, are also often found in the diet of large individuals of silver bream. Being a food competitor of bream, this species is able to feed on highly mineralized soils, including zebra mussel colonies (Malinin et al., 1990; Gerasimov, 2015). Figure 3b reflects the currently observed improvement in the size and age characteristics of the silver bream in comparison with the data from 1980s. Apparently, as a result of changes in the composition of the benthic community and, as a consequence of this, the conditions benthic feeding fish, the number of the population of silver bream also slightly increased in the lake. If the share of this species averaged about 6–10% in catches of the early 1980s, it currently ranges from 4 (spring) to 25% (autumn) in the littoral and sublittoral zones (Fig. 2). At the same time, the growth of another benthophagous species – bream has slowed somewhat (Fig. 3c). This is even more surprising because, as previously shown, the abundance of the main bream’s food organisms (oligochaetes and chironomids) increases markedly in the presence of zebra mussel colonies (Jernelöv, 2017). It is possible that the deterioration of trophic conditions for this species is due to the formation of expansive fields of dead shells in the littoral and sublittoral zones of the lake that impede the consumption of soft zoobenthos by the bream (Nikitenko, Shcherbina, 2014). This is consistent with the data of other researchers which showed that the high environment-forming activity of D. polymorpha associated with the creation of druse conglomerates changes the availability of other benthic organisms for fish (Yakovleva, Yakovlev, 2011; Mayer et al., 2013). According to our assumptions, large bream individuals are forced to stay on the shell-free parts of the bottom, during the feeding season (Fig. 4c–4d). As for the morphology of the pharyngeal teeth of bream and silver bream, the introduction of zebra mussel into the lake did not cause processes similar to those in observed in roach populations, according to our data.
To date, the reasons for a significant reduction of ruffe catches in Lake Plescheevo remain not fully understood. Earlier (the beginning of the 1980s) its stocks in the reservoir were comparable with those of bream and silver bream, but according to catches of 2014–2016 they were significantly inferior to them (Fig. 2). Perhaps the decrease in the number of this species is explained by its increased vulnerability to oxygen deficiency in the bottom layer of water. The tendency towards an increase in water temperature in temperate latitudes that has been observed in recent decades (Lazareva, 2018), as well as the development of specific algal flora in Lake Pleshcheevo (Sakharova, 2019), can lead to an earlier occurrence and a longer period of acute oxygen deficiency in the bottom layer of water. Apparently, this significantly worsens the situation with summer kills in the ruffe population. Moreover, as shown by North American researchers, zebra mussels can cause increases in toxic blue-green algae (Snyder et al., 1992; Jernelöv, 2017). Decaying algae can make waters uninhabitable by causing low levels of dissolved oxygen that result in fish kills.
Despite significant differences in the methods for assessing the relative abundance and species composition of catches of the past (early 1980s) and present (2014–2016), a marked tendency toward a change in the ratio of fish of pelagic and benthic complexes can be noted. Similar processes were previously discussed in detail by Mayer et al. (2013) using a series of lakes of North America and Europe located in the temperate climate zone. The term “benthification” they proposed reflects the essence of the changes occurring in lake ecosystems. The consequences of the introduction of zebra mussel affect not only certain trophic relationships in communities, but also cause large-scale multi-level changes in the structure of the entire ecosystem through large-scale filtration activity of the mollusk (Mayer et al., 2013). Removing a significant portion of the organic and mineral suspensions from the water, zebra mussel contributes to a significant decrease in the trophicity of water bodies (Mackie, Schloesser, 1996; Shcherbina, 2009). Therefore, relocating nutrients from the water column to the bottom, thereby depleting the pelagic and enriching the benthic community (Mayer et al., 2013; Jernelöv, 2017). In addition, “wherein increased water clarity (a physical alteration) triggers a predictable suite of modifications to ecosystem structure (e.g., species composition, spatial distribution of primary producers and consumers) and function (e.g., primary production, benthic – pelagic flux)” (Mayer et al., 2013, p. 576).
However, the formation process in Lake Pleshcheevo biocenosis of D. polymorpha coincided in time with a period of a general decrease in the anthropogenic load on this water body. The foundation in 1988 of the Pereslavl natural-historical national park (currently the Pleshcheevo Lake National Park), the associated fishing restriction, as well as the depression of agriculture in the 1990s, which led to a decrease in the supply of nutrients from the catchment area, probably had no less impact on the ecosystem of the lake. Apparently, not only the introduction of zebra mussel, but a combination of the above factors caused the structural changes described above in the fish part of the lake community.
CONCLUSION
Presented own and literary material allows us to conclude that the occurrence of a sustainable biocenosis of D. polymorpha in Lake Plescheevo involved processes that directly and indirectly determine the structure and spatial distribution of the fish community. According to our estimates, the role of benthophagous fish has significantly increased in the lake, which was a direct consequence of the change in the food value of macrozoobenthos and the environment-forming activity of this mollusk. At the same time, the size and age characteristics have noticeably improved in populations of certain species (roach, silver bream), while deteriorating for other (bream). Data from this study showed that changes also affected the pelagial of the lake. Thus, small roach, earlier numerous in pelagic aggregations, is now practically completely absent. Apparently, this event is due to an improvement in the feeding conditions for the species in the littoral and sublittoral zones of the lake on the one hand, and an increase in food competition with aboriginal pelagic species (vendace, bleak) on the other. Further observations would allow for a more detailed study of the trends discussed in the present work on the transformation of the lake ecosystem and can help preserve this unique landscape object in the European part of Russia.
Список литературы
Боровикова Е.А., 2017. Особенности генетической структуры и происхождения популяции ряпушки Coregonus albula озера Плещеево // Известия Российской академии наук. Серия биологическая. № 3. С. 228–233. [Borovikova E.A., 2017. Special traits of the genetic structure and origin of the population of vendace Coregonus albula of Pleshcheyevo Lake // Biology Bulletin. V. 44. P. 245–250].
Bakanov A.I., 1983. Bentos of Pleshcheevo Lake // Proceedings of IBVV AN USSR. V. 51. P. 70–83. [In Russian]
Borovikova E.A., Artamonova V.S., 2018. Morphological specificities of vendace (Salmoniformes: Salmonidae: Coregoninae: Coregonus albula) population in Lake Pleshcheyevo (the Volga River basin): relationships of two phylogenetic lineages in a new zone of secondary contact // Organisms Diversity and Evolution. V. 18. P. 355–366.
Brabrand A., Faafeng B., 1993. Habitat shift in roach (Rutilus rutilus) induced by pikeperch (Stizostedion lucioperca) introduction: predation risk versus pelagic behavior // Oecologia. V. 95. P. 38–46.
Butorin N.V., Sklyarenko V.L. (eds), 1989. Pleshcheevo lake ecosystem. Leningrad: Nauka. 264 c. [In Russian]
Chugunova N.I., 1963. Age and growth studies in fish: a systematic guide for ichthyologists. Washington: National Science Foundation, by the Israel Program for Scientific Translations. 132 p.
Dgebuadze Yu.Yu., Petrosyan V.G., Khlyap L.A. (eds), 2018. The most dangerous invasive species of Russia (TOP-100). M.: KMK Scientific Press. 688 p. [In Russian]
Eklov P., 1997. Effects of habitat complexity and prey abundance on the spatial and temporal distributions of perch (Perca fluviatilis) and pike (Esox lucius) // Canadian Journal of Fisheries and Aquatic Sciences. V. 54. P. 1520–1531.
Ivanter E.V., Korosov A.V., 2003. Introduction to quantitative biology. Petrozavodsk: PetrSU. 304 p. [In Russian]
Gerasimov Yu.V. (ed.), 2015. Fishes of the Rybinsk reservoir: population dynamics and ecology. Yaroslavl: Filigran. 418 c. [In Russian]
Gerasimov Yu.V., Malin M.I., Borisenko E.S., Zhdanova S.M., Tsvetkov A.I., Smirnov A.K., 2019. Foraging behavior and feeding of vendace (Coregonus albula) in Lake Pleshcheyevo during the period of temperature stratification // Lakes of Eurasia: problems and solutions. Kazan: AN RT. P. 234–239. [In Russian]
Jernelöv A., 2017. Zebra Mussels in Western Europe and North America // Jernelöv A. The Long-Term Fate of Invasive Species. Cham, Switzerland: Springer. P. 11–30.
Kasyanov A.N., Izumov Yu.G., 1995. Studies of the growth and morphology of the roach Rutilus rutilus in the Lake Pleshcheevo in the connection with introduction of the Dreissena // Voprosy Ikhtiologii. V. 35. P. 546–548. [In Russian]
Kasyanov A.N., Yakovlev V.N., Izyumov Yu.G., Zhgareva N.N., 1981. Variability of pharyngeal teeth of the roach Rutilus rutilus (L.) depending on the type of feeding // Voprosy Ikhtiologii. V. 21. № 4. C. 595–599. [In Russian]
Kodukhova Yu.V., 2011. Yearly variations of impact of natural hybrids of bream and roach (Abramis brama (L.) × × Rutilus rutilus (L.)) in Rybinsk Reservoir // Russian Journal of Biological Invasions. V. 2. P. 204–208.
Kodukhova Yu.V., Karabanov D.P., 2017. Morphological changes in the population of roach (Rutilus rutilus, Cyprinidae) in Lake Pleshcheevo as a result of the introduction of the mollusk, Dreissena polymorpha (Bivalvia) // Zoologicheskiy Zhurnal. V. 96. P. 1069–1077. [In Russian]
Lazareva V.I. (ed.), 2018. Structure and functioning of the ecosystem in the Rybinsk reservoir at the beginning of the 21st century. Moscow: Russian Academy of Science. 456 p. [In Russian]
Mackie G.L., Schloesser D.W., 1996. Comparative biology of Zebra mussels in Europe and North America: An overview // Amer. Zool. 36. P. 244–258.
Malinin L.K., Kiyashko V.I., Linnik V.D., 1990. Ecological differentiation of feeding aggregations of bream // Proceedings of IBVV AN USSR. V. 60. P. 23–37. [In Russian]
Malinin L.K., Linnik V.D., 1983. The density and spatial distribution of mass fish species in Lake Pleshcheevo // Proceedings of IBVV AN USSR. V. 51. P. 125–159. [In Russian].
Mayer C.M., Burlakova L.E., Eklöv P., Fitzgerald D., Karatayev A.Y. et al., 2013. Benthification of Freshwater Lakes Exotic Mussels Turning Ecosystems Upside Down // Nalepa T.F., Schloesser D.W. (eds). Quagga and Zebra Mussels. Biology, Impacts, and Control, Second Edition. Boca Raton, USA: CRC Press. P. 575–585.
Nalepa T.F., Schloesser D.W., 2013. Quagga and Zebra Mussels. Biology, Impacts, and Control, Second Edition. Boca Raton, FL: CRC Press. 815 p.
Nikitenko E.V., Shcherbina G.Kh., 2014. Feeding of the bream of the Chograisk reservoir // Bulletin of IIRAT. V. 2. P. 57–62. [In Russian]
Persson L., 1993. Predator-mediated competition in prey refuges: the importance of habitat dependent prey resources // Oikos. V. 68. P. 12–22.
Pravdin I.F., 1966. Guide for the study of fish. Moscow: Pishchevaya promyshlennost. 376 p. [In Russian]
Pryanichnikova E.G., 2019. Taxonomic composition of macrobenthos lake Plescheevo // Transactions of Papanin Institute for Biology of Inland Waters Russian Academy of Science. V. 86. P. 57–71. [In Russian]
Pryanichnikova E.G., Tsvetkov A.I., 2018. Main characteristics of the Lake Pleshcheyevo population of Dreissena polymorpha (Bivalvia, Dreissenidae) // Ecosystem Transformation. V. 1. P. 11–18. [In Russian]
Reshetnikov Yu.S. (ed.), 2010. Fishes of the Russian nature reserves. Volume 1. Freshwater fishes. Moscow: KMK Scientific Press. 627 p. [In Russian]
Sakharova E.G., 2019. The phytoplankton of lake Plescheevo in 2014–2016 // Transactions of IBIW RAS. V. 86. P. 23–33. [In Russian]
Shcherbina G.K., 2008. The structure of Dreissena polymorpha (Pallas) biocenosis and the role of mollusk in roach Rutilus rutilus (Linnaeus) feeding in Pleshcheevo lake // Inland Water Biology. V. 1. P. 380–387.
Shcherbina G.Kh., 2009. Changes in species composition and structural and functional characteristics of macro-zoobenthos of aquatic ecosystems of the North-West Part of Russia under the influence of natural and anthropogenic factors. Extended abstract of doctoral biology dissertation. St.-Petersburg: Limnology Institute Russian Academy of Science. 468 p. [In Russian]
Smirnov A.K., Smirnova E.S., Koduhova Yu.V., Karabanov D.P., 2019. Tolerance of juvenile perch Perca fluviatilis and amur sleeper Perccottus glenii to predation by pike Esox lucius // Journal of Ichthyology. V. 59. P. 382–388.
Smokorowski K.E., Pratt T.C., 2007. Effect of a change in physical structure and cover on fish and fish habitat in freshwater ecosystems – a review and meta-analysis // Environmental Reviews. V. 15. P. 15–41.
Snyder F.L, Garton D.W., Brainard M., 1992. Zebra mussels in the Great Lakes: the invasion and its implications. Columbus: Ohio Sea Grant College Program.
Stolbunov I.A., 2008. Morphofunctional differences of roach (Rutilus rutilus) in Plescheevo lake // Biosystems Diversity. V. 16. P. 191–196. [In Russian]
Stolbunova V.N., 2006. Zooplankton of Lake Plescheevo. Moscow: Nauka. 152 p. [In Russian]
Strelnikov A.S., Permitin I.E., 1983. Ichthyofauna of Lake Plescheevo and fisheries condition // Proceedings of IBVV AN USSR. V. 51. P. 97–112. [In Russian]
UNDED, 2012. United Nations Declaration on Environment and Development. AGENDA 21. United Nations Sustainable Development Knowledge Platform [Internet]. Accessed at: https://sustainabledevelopment.un.org/content/ documents/Agenda21.pdf. 351 p.
Yakovleva A.V., Yakovlev V.A., 2011. Impact of Dreissena polymorpha and Dreissena bugensis on structure of zoobenthos in the upper reaches of the Kuybyshev Reservoir (Russia) // Russian Journal of Biological Invasions. V. 2. P. 312–319.
Zhdanova S.M., 2018. Veliger larvae of Dreissena (Bivalvia, Dreissenidae) in the zooplankton of Lake Pleshcheyevo (Yaroslavl Region, Russia) // Ecosystem Transformation. V. 1. P. 19–29.
Zhdanova S.M., Sabitova R.Z., Tsvetkova M.V., 2019. Composition and structure of the zooplankton community in lake Pleshcheyevo // Transactions of Papanin Institute for Biology of Inland Waters, Russian Academy of Science. V. 86. P. 34–56. [In Russian]
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


