Зоологический журнал, 2022, T. 101, № 12, стр. 1429-1438
The survivability of individuals within a tiger (Panthera tigris) subpopulation in the Lazovsky Nature Reserve and Zov Tigra National Park, Russian far east
G. P. Salkina a, *, N. Ya. Poddubnaya b, S. A. Kolchin c, V. S. Kolesnikov d
a FGBU “Joint Directorate, L.G. Kaplanov Lazovsky State Nature Reserve and “Zov Tigra National Park”
692980 Lazo, Russia
b Cherpovets State University
162600 Cherepovets, Russia
c Institute of Water and Ecology Problems, Far East Branch, Russian Academy of Sciences
680000 Khabarovsk, Russia
d Tiger Protection Society
692980 Lazo, Russia
* E-mail: tpsrus@mail.ru
Поступила в редакцию 17.05.2022
После доработки 5.08.2022
Принята к публикации 6.08.2022
- EDN: SMBVMH
- DOI: 10.31857/S0044513422120108
Аннотация
From 2011 to 2020, tigers were recorded using camera traps in the Lazovsky Nature Reserve and Zov Tigra National Park, Southeast of the Sikhote-Alin Mountains. Sixty-seven adult or subadult individuals were identified. On average each year, 14 individuals were photographed in the Nature Reserve, and 8 were photographed in the National Park. The number of tigers recorded in the Nature Reserve appears to be declining, but there are no significant trends of this sort observed in the National Park. The tigers’ survival rate was calculated for 10 one-year intervals. The analysis included 32 males and 27 females from the group of adults and subadults, whose disappearances were unlikely to be connected with natural dispersal. The vast majority were detected for just 1–2 years. The survival rate for tigers of both sexes decreased across the 10 time intervals, particularly sharply between the first and second. In years 4–6, the survival rate for the females was lower, with a sharp decline observed at the start of the fourth year. During the observation period, at least seven deaths of tigers have been reported on the research site and in the vicinity (Lazovsky district in the Primorsky Krai region), six of which were killed by poachers. This unnatural turnover of adult and subadult tigers within this group in protected areas can be explained by poaching in the neighboring, unprotected areas. The creation of an extensive buffer zone with a ban on hunting around the Nature Reserve and National Park has been proposed to address threats from poaching.
At the International Tiger Summit, held in 2010 in St Petersburg, the leaders of countries with extant tiger populations pledged to double tiger numbers by 2022 (Global …, 2010). In order to achieve such a goal, it is important to assess the survival rate of the individuals in the population, which determines its size and the potential for future growth.
In recent years, digital camera traps have been widely used to study the ecology of tigers and other mammals (Karanth, Nichols, 1998, 2002; Soutyrina et al., 2013; Riley et al., 2017; Salkina et al., 2019, etc.). Thanks to the ability to identify specific tigers from photographs, researchers are able to track the fate of individuals over many years and study the turnover of tigers within a particular group. By turnover, we mean the disappearance of some tigers and the appearance of others. Obviously, this process is primarily regulated by reproduction and mortality of resident tigers along with the dispersal of younger ones.
We use mortality as an indicator to reflect the number of deaths of individual tigers in a group over a certain period of time (Chernova, Bylova, 1981). The survival rate is the proportion of animals that are still alive after a certain period. In population ecology, in order to study patterns of mortality (and accordingly, survival), cohort and static tables of survival are compiled (Bigon et al., 1989). To analyze the data in such tables, it is necessary to know the ages of the individuals. To compile the cohort tables, you need to determine the proportion of individuals that have died in each age group. For static tables, data is needed on the number of individuals in each age group of the overall group being studied. In the wild, it is difficult to obtain the necessary data for populations of certain animals, such as tigers and especially the Amur subspecies (P. t. altaica Temminck 1844), whose population density in the north of their species range is naturally low. The photo-registration of tigers makes it possible to study the turnover of individuals in particular groups across certain time intervals. During each of these intervals, some individuals disappear while others remain. In the survival tables for this type of analysis, it is not the age of the individuals that is taken in consideration, but the time interval in which an individual either disappeared or continued to be recorded. Analysis of these tables provides an estimated survival rate (Khalafyan, 2007).
The survival rate of the Amur tiger population has been poorly studied. To determine the survival rate in the groups chosen for study, it has been necessary to identify individuals and to exclude those tigers who may have disappeared for reasons other than death, as well as to study the differences in the survival rates of males and females. To implement all this, we conducted a photo-registration of tigers on the territory of the Lazovsky Reserve and the Zov Tiger National Park from 2011 to 2020. According to the photographs recorded, 67 adult and subadult individuals were identified and their presence in reserves was determined. Based on the data documenting turnover of individuals, the survival rate of tigers was calculated and measures for further tiger conservation were proposed.
MATERIALS AND METHODS
Study area. The research was carried out in the Southeast of the Sikhote-Alin Mountains, specifically in the Lazovsky Reserve, Zov Tigra National Park, and in the vicinity of these sites around the basins of neighboring rivers (Fig. 1). The Lazovsky Reserve was established in 1935 and currently encompasses 1210 km2. Zov Tigra National Park was created in 2007 on an area 833 km2 in size. Both sites are comprised of typical Amur tiger habitat (mountainous Korean pine – broadleaved forests) and together they form the main reproductive core of the Amur tiger population in the southeastern Sikhote-Alin Mountains. In these protected areas, hunting, logging, mining, and any other activity that harms wildlife habitat, is prohibited.
There are no protective buffer zones around the Lazovsky Reserve and Zov Tigra National Park. There are four hunting grounds in the immediate vicinity of the Reserve and five around the National Park (Atlas …, 2004). Along the border of the Reserve, vast fields of crops are grown to attract animals from the protected areas. Due to the sharply indented borders of the Reserve, the fields run deep into these territories. These cultivated fields are visited by wild boars (Sus scrofa L. 1758), sika deer (Cervus nippon Temminck 1838), red deer (Cervus elaphus L. 1758), roe deer (Capreolus capreolus L. 1758), Asiatic black bears (Ursus thibetanus G. Cuvier 1823) brown bears (Ursus arctos L. 1758), and tigers. Numerous hunting towers have been built in these fields (Salkina, Kolesnikov, 2010; Salkina, 2013), and bait is laid out to attract ungulates, bears, and tigers. Thus, the animals from the reserves become the target of hunting and poaching.
Photo-registration and survival rate of the tigers. In the Lazovsky Reserve, the photo-registration of tigers was carried out between 2011–2020 and, in the Zov Tigra National Park, between 2015–2020. Cameras were installed at a minimum of every 50 km2 in places the tigers were most likely to frequent: focused on trees and rocks often used by tigers for scent-marking (Salkina et al., 2017). Of the cameras deployed from December to May, 30–40% of them worked all year round. Individuals were identified using the pattern of stripes and spots on their flanks, which are unique to each tiger (Karanth, Nichols, 1998). The subadult tigers were difficult to distinguish from the adults, so these two categories were combined into one age group. Tiger cubs under the age of one differ significantly in body size from individuals in the older age group.
In addition to the camera survey, any tigers that died in the vicinity of the study area (the Lazovsky District of the Primorsky Krai) were identified and recorded. The pattern of spots and stripes on the bodies or the pelts were compared with the images of the tigers that were available in our archive. The authors were involved as experts and witnesses in criminal cases relating to the confiscation of skins and other tiger derivatives, trophy photographs, and the wounding and subsequent deaths of tigers. In a number of cases where our archive lacked photographs of certain tigers that had been killed, the location of the tigers’ deaths was established using the trophy photographs, in which recognizable elements of the landscape had been captured.
The data was processed using Statistica 10 software, specifically, the Survival Analysis module (Statsoft, 2010). This module allows you to use, not only the data relating to the individuals that have disappeared (“complete data”), but also the data on the individuals that continue to be recorded (“incomplete data”). One calendar year was used as the time interval and a total of 10 intervals were studied (from 2011 to 2020). In 2011, observations were not carried out for the full year and in 2012, only a limited number of camera traps were deployed. Therefore, these years were not taken into account when calculating the total annual number of tigers recorded. Naturally, the six-month period cameras were predominantly operational affected the number of tigers detected. However, detection of resident individuals and their survival, which is of greatest interest, was not significantly affected.
The analysis of survival excluded cubs that were under a year old, as well as any cubs that became subadults and disappeared at this age. This is because the factors that lead to the disappearances of these tigers (infant death, natural dispersal, etc.) can differ significantly from individuals in the adult group.
Using the Survival Analysis module, the number and proportion of “living” and “disappeared” individuals were calculated for each of the time intervals (Table 1). The table includes the following columns: “number exposed” is the number of studied individuals or number of individuals that were “living” at the beginning of the time interval under consideration, minus half of the disappeared, “proportion of disappearances”, which is the ratio of the number individuals that disappeared during a given interval to the total number of individuals being studied during that interval (if there are no disappeared individuals in this time interval, then the number 0.5 is divided by the number of studied individuals. This procedure is necessary in order for the evaluation of the survival function to be continuous); the “proportion of those that remain” is equal to one, minus the proportion of disappearances; the “cumulative proportion of those that remain” is the cumulative proportion of those that remain at the start of the corresponding interval (Khalafyan, 2007). Since the probabilities of survival (and not “disappearing”) are considered independent for each interval, the cumulative proportion is equal to the product of the proportion of individuals that survive (remain) across all previous intervals. The resulting value as a function of time is also called “survival”, or the “survival function”. The survival function is an estimate of the probability that an individual will “survive” during a given interval (Khalafyan, 2007).
Table 1.
Survival rates of tigers in the Lazovsky Reserve and Zov Tigra National Park between 2011–2020
| Interval in years | Number of individuals recorded at start of interval | Number exposed | Number of individuals that disappeared during given interval | Proportion of disappear-ances | Proportion of those remaining | Cumulative proportion of those remaining (surviving) | Standard error cumulative survival |
|---|---|---|---|---|---|---|---|
| 1 | 59 | 56.0 | 18 | 0.321429 | 0.678571 | 1.000000 | 0.000000 |
| 2 | 35 | 32.5 | 8 | 0.246154 | 0.753846 | 0.678571 | 0.062409 |
| 3 | 22 | 20.5 | 4 | 0.195122 | 0.804878 | 0.511538 | 0.069588 |
| 4 | 15 | 15.0 | 3 | 0.200000 | 0.800000 | 0.411726 | 0.071706 |
| 5 | 12 | 9.5 | 1 | 0.105263 | 0.894737 | 0.329381 | 0.071407 |
| 6 | 6 | 4.5 | 0 | 0.111111 | 0.888889 | 0.294709 | 0.071816 |
| 7 | 3 | 3.0 | 0 | 0.166667 | 0.833333 | 0.261964 | 0.077339 |
| 8 | 3 | 3.0 | 0 | 0.166667 | 0.833333 | 0.218303 | 0.085620 |
| 9 | 3 | 3.0 | 1 | 0.333333 | 0.666667 | 0.181919 | 0.085423 |
| 10 | 2 | 1.5 | 1 | 0.666667 | 0.333333 | 0.121280 | 0.075463 |
To compare the survival rates of the males and females, the Gehan-Wilcoxon test, Cox-F test, Cox-Mantal test, Peto generalised Wilcoxon test, and the log-rank test were used.
RESULTS
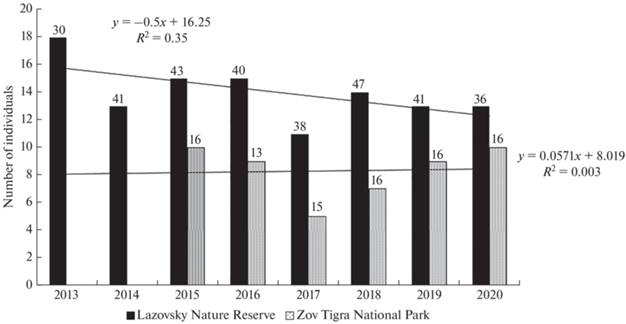
Within the study area, a total of 67 adult and subadult tigers and 44 tiger cubs were identified from photographs. Some of the animals initially detected as cubs and subadults were classified as adults as they aged in subsequent years. Every year in the Reserve (since 2013), between 11 and 18 adults and subadults were photographed, an average of 14; in the National Park (since 2015), between 5 and 10 were photographed, an average of 8 (Fig. 2). The number of individuals registered annually did not depend on the number of camera traps used. Some of the tigers were sighted in both areas during similar time intervals. The analysis of the survival rates did not include 7 cubs that transitioned into the subadult group, but who were detected for no more than around 3 years. Their disappearance could be associated with natural dispersal. The analysis included 3 full-grown tiger cubs that had been registered for over 3 years; at this age, young tigers can transition into the category of resident tigers, as they go on to establish their own home ranges.
Fig. 2.
The number of adult and subadult tigers photographed in the Lazovsky Reserve and Zov Tigra National Park from 2013 to 2020 and the linear trends of this indicator. The numbers above the columns indicate the number of camera traps.
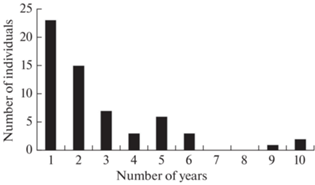
Thus, the survival analysis included 59 adults and subadults, including 32 males and 27 females. Individuals from this group were detected for between 1 to 10 years, the average duration being 2.5 years. In the Reserve between 1 and 7 individuals disappeared each year (failed to appear the following year), and in the National Park, between 0 and 5. Most of the individuals were recorded for only 1–2 years (Tabl. 1; Fig. 3).
Fig. 3.
Duration of recording (calendar years) of adult and subadult tigers in the Lazovsky Reserve and Zov Tigra National Park between 2011–2020.
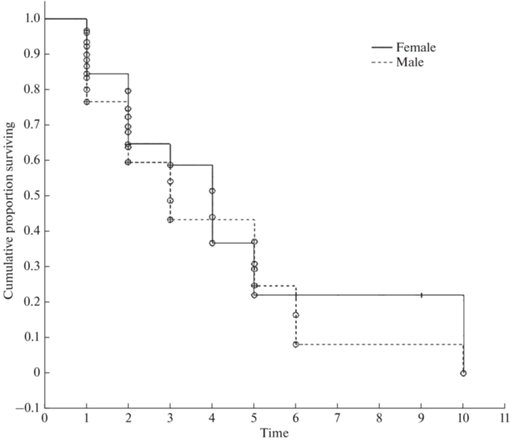
Males were observed for, on average, 2.2 years, and females, for 3.3 years. The probabilities from all five of the tests through which the survival rates of the males and females were compared were much higher than the 0.05 significance level used by us. This means there is no significant difference between survival rates for p < 0.05. Furthermore, for some time intervals, the survival rate of the females was even lower than that of males (Fig. 4).
Fig. 4.
Survival curves of female and male tigers in the Lazovsky Reserve and Zov Tigra National Park between 2011–2020 (The circles indicate the individuals that have disappeared, and the plus-signs stand for those that have remained).
From 2011 to 2020 at the study site and in its vicinity (the Lazovsky District of the Primorsky Krai) at least 7 adult and subadult tigers died, 6 at the hand of poachers (2 females, 1 male, 3 presumed males), and 1 male that drowned. As part of the criminal investigation, we examined five different tigers, using the skins of two tigers provided by the police and trophy photographs containing images of three tigers that had been killed, including an adult female and a male that had been living in the Lazovsky Reserve. Another tiger, probably a male, had been killed adjacent to the Reserve. In another case, a subadult female had been wounded by a poacher at the border of the Reserve and subsequently died.
DISCUSSION
The number of tiger cubs we photographed turned out to be undercount, which was determined by counting their tracks in winter (Salkina, Kolesnikov, 2010; Kerley, Borisenko, 2017). The fact is that female tigers with litters stay within a limited area, often avoiding travel corridors used by other adult tigers. Accordingly, cubs are not always detected by camera traps (Kolchin, Soutyrina, 2012; Soutyrina et al., 2013; Matiukhina et al., 2016; Lukarevskiy et al., 2021). Moreover, not all cubs in a litter are reliably photographed, even when they are travelling in a line, one after the other. This is due to periodic pauses between exposures when camera traps take more than one image. Most of the cubs were detected in their first year of life and then less often in their second, due to the nature of tiger reproduction and sociality, and to the mortality rates of tiger cubs. Tigers enter independence at 20–22 months of age (Yudin, Yudina, 2009). At this time, the young begin to separate and resettle themselves and, with few exceptions, leave the mother’s home range. A female tiger has a new offspring 2+ years after the birth of the previous litter, if the cubs of the first litter survived.
Certainly, the mortality rate of tiger cubs is high for natural reasons (Yudin, Yudina, 2009). The disappearance of subadult tiger cubs may be connected with death or dispersal. First of all, cubs are vulnerable to risks specific to their size and age, but we do not have enough data to analyze this. Secondly, in terms of the survival (mortality) rate, those individuals that have disappeared due to dispersal cannot be taken into account. For these reasons, we did not include any young tigers in our analysis that were recorded less than three years after their birth.
In the Lazovsky Reserve, the number of tigers photographed annually trended downwards over the course of our study; in the Zov Tigra National Park there are no significant trends apparent (Fig. 2). Over the same period, the indicator of the relative abundance of tigers (track density) in the reserve did not show a significant downward trend, although the amplitude of its fluctuations has increased in recent years (Salkina et al., 2018). This indicates instability in this tiger group, which is, most likely, related to the frequent turnover of individual tigers.
Undoubtedly, not all tigers visiting the study areas throughout the year can be recorded with the camera traps. During the snow-free part of the year only a limited number of cameras were used and the probability of detecting tigers depends on their location (Salkina et al., 2017). Therefore, camera traps likely undercount the total number of tigers that have visited the Reserve and the National Park. This is particularly true in relation to transient tigers that only visit the study areas temporarily.
The survival function of the tigers drops sharply from the first to the second year of their being detected in the reserves (Table 1). This indicator decreases more gradually in subsequent years. The standard errors of the estimates of this function turned out to be smaller than for the estimates themselves, indicating their reliability. Survival estimates are based on the proportion of surviving and disappeared individuals. However, the disappearance of individuals may not always be connected with their deaths. Transient individuals could be detected in a given year, for example. However, it is unlikely that there are many of these types of individuals within the population. In Chitwan National Park (Nepal), long-term studies have shown that transient individuals accounted for 7% of the total number of tigers living there, including cubs (Barlow et al., 2009).
Over the course of a 1–2-year period, subadults born in the Reserve or National Park who eventually disperse may be observed, but it may be that the cameras did not detect them when they were living with their mother, or they may have been born outside of the study areas. The photo registration of the tigers showed that, when resident individuals disappear, neighboring tigers begin to visit the vacant territories (our unpublished data). Such tigers can be observed visiting the territories at different intervals over the following 1–2 years, sometimes disappearing for a year or more. Their further disappearance could be connected with the arrival and establishment of new resident tigers. Thus, the actual survival rate of tigers in the period under consideration, specifically for the first two years, is likely to be higher than our estimate.
It is likely tigers that established home ranges, then disappeared in their first or second year of being in the reserves, probably died. The subsequent appearance of neighboring tigers in the vacated areas, rather than the appearance of new individuals, may indicate that the most likely reason for the disappearance of the tigers was death rather than territorial conflicts and expulsion by conspecifics. In the Sikhote-Alin Reserve, changes in territory occurred mainly after the death of a resident tiger, usually as a result of poaching. In this area, the displacement of a resident tiger by an immigrant individual has not been observed (Goodrich et al., 2010). In the Lazovsky Reserve, such incidents are known (our unpublished data), but similar cases elsewhere are rare. In Nepal, resident females did not use vacated territories as part of their home ranges (Smith et al., 1987), although females with cubs did expand their territories (McDougal, 1977). In this region, vacant territories were inhabited by young individuals who had either dispersed there or who had remained close to their maternal home range (Smith et al., 1987; Smith, 1993). Based on this, and taking into account the fact that the proportion of transient tigers in the population may be small, we can conclude that if we are underestimating the survival rate of the tigers at all during the initial 1–2-year intervals, it is not by much.
The survival rate of the tigers in the intervals after the second year of being detected, which also shows a trend of steady decline, in our opinion, accurately reflects the situation in terms of mortality for individuals in the study areas (Table 1). There are only two tigers (a male and a female) that have been recorded in the Reserve for 10 years, though there is another female who is 9 years old. In the wild, the maximum known age of an Amur tiger is 19 years (Yudin, Yudina, 2009). The home ranges of long-lived tigers include the Sea of Japan coastline, which has practically no border with the adjacent hunting territory comprising vast fields of crops (Salkina et al., 2019). The long-lived male’s home range covered around half of the area of the Reserve. As mentioned above, in 2018, a new male appeared here, and the old one moved to the center of the Reserve. According to our data, the long-lived females, unlike the other female tigers in the Reserve, gave birth to offspring almost every two years. Thus, the survival rate and reproductive output in these females turned out to be higher than for female tigers living near hunting areas. In any case, the home ranges of almost all of the tigers extended beyond the borders of the protected areas.
The period that females remained in the Reserve and in the National Park was, on average, longer than that of males. Within most time intervals, the female survival rate was also higher (Fig. 4). However, the differences in survival rate have not been statistically confirmed. Moreover, for the intervals between 4–6 years, the survival rate for males was higher, while the survival rate for females for this period decreased sharply. Female Amur tigers become sexually mature at the age of 3.5 years (Yudin, Yudina, 2009). It is most likely that a sharp decrease in their survival rate, upon reaching 4 years of age, is associated with their first mating and litter. Females travel long distances during estrus in search of mates (our unpublished data). According to V.G. Yudin and E.V. Yudina (2009), females also often move before parturition, and are restless in the den with tiger cubs. Females become more vulnerable, including to poachers, because they are more likely to engage with a person for a longer period of time, trying to protect their cubs. In addition, feeding tiger cubs requires females to move more often and visit anthropogenic landscapes, which may increase the risk of encountering poachers. Having gone through a critical period of a tiger’s life cycle, rearing their first litter, the female tigers have acquired valuable experience that increases their chances of survival when rearing future litters. The early deaths of females significantly slows down the reproductive output for the tiger population overall.
In the Primorsky Krai region between 1972 and 1992, out of the 55 adult tigers found dead, there were twice as many males as females (Nikolaev, Yudin, 1993). Between 1983 and 2009, males also dominated the overall number of adult and subadult tigers that died in the Primorsky and Khabarovsk regions (Salkina, 2010). This phenomenon must be assessed with the sex ratio within the tiger population taken into account. According to the winter track records data in Amur tiger range, on average, the proportion of adult and subadult females is higher than that of males. However, according to the data of our long-term photographic registration, the sex ratio was practically equal, although in some years it can change slightly. Obviously, the sex of the tigers can be determined more accurately from photographs than from tracks. In any case, males accounting for twice the number dead individuals compared to females indicates a higher mortality rate among males in the Amur tiger population.
The heightened mortality of males can be explained by their ecology and behavior. Young female tigers are more philopatric than males and, after separation from their mother, may still occupy part of the maternal home range (Smith, 1993; Goodrich et al., 2008). Males establish themselves within home ranges that are two or more times larger than the home ranges of females (Salkina, 1993; Salkina, Solomkina, 2005), and during dispersal they can travel considerable distances from their birth site. They walk further, show less caution, hunt domestic animals more often and, accordingly, are involved in conflict situations with humans more often (Salkina, 2011). In the Sikhote-Alin Reserve, the annual survival rate of adult females was higher than that of adult males (Goodrich et al., 2008).
From looking at survival rates, any bias in mortality towards males in the group of tigers in our study is not quite as apparent. Females are less likely to attack domestic animals and, therefore, are less likely to interact with humans. However, when seeking ungulates, female tigers are known to actively visit the cultivated fields bordering protected areas. In addition, we found meat laid out in these areas to attract Asiatic black bears, brown bears, and tigers. The meat was typically carcasses of wild and domestic animals or parts thereof. Shooting animals in these fields is carried out from numerous hunting towers. Two female tigers were killed by poachers directly on the fields baited with meat. In 2021, a group of poachers specializing in shooting tigers and selling their derivatives on the Chinese black market was detained in the Lazovsky District of the Primorsky Krai.
The cases of tiger deaths during our research were recorded mainly in territory adjacent to the Lazovsky Reserve. Between 1947 and 2009, due to human actions, 44 of 56 (79%) tigers died in the Lazovsky District of Primorsky Krai, and half of these were poached (Salkina, 2010). In the past decade, of the eight reported tiger deaths in this area, only one has died of natural causes while the other seven died as a result of poaching.
A study by Skidmore (2021) showed that tightening up criminal penalties in the Russian Federation for killing tigers or manipulating their derivatives did not decrease levels of tiger poaching. The shift in the law only changed the nature of illegal derivatives trading. It moved it underground, strengthening the ties between those involved and, as a result, the activity actually became more covert and efficient. Thus, poaching is still the main cause of death for the Amur tiger across its entire range in Russia. We, therefore, associate the disappearance of the majority of the resident tigers in the study area with this activity.
The frequency of turnover of individuals in the groups of tigers in the Lazovsky Reserve and in Zov Tigra National Park is unnatural and indicates high mortality and instability within the population. These characteristics have recently been confirmed by analysis of the tiger tracks recorded during the winter census of Amur tigers (our unpublished data). A similar situation was observed in the Sikhote-Alin Reserve, however, the unstable nature of that group was connected to a decrease in population (Soutyrina et al., 2014). The most intense rate of change of individuals occurred in the southern part of Sikhote-Alin Reserve, where human impact is greater. The frequent turnover of tigers in our study area is associated with poaching in adjacent areas and, accordingly, with the inadequacy of protective measures. Of particular concern is the high mortality rate of the females that have reached reproductive age.
According to paragraph 3, Article 78 of the Land Code of the Russian Federation: “The use of agricultural land permits the implementation of hunting activities, unless otherwise stated by this Code.” However, in paragraph 4 of this article it states: “Land plots from agricultural land located at a distance of no more than thirty kilometers from the borders of rural settlements cannot be used for purposes that are unrelated to agriculture.” Since almost all agricultural fields bordering the Lazovsky Reserve and Zov Tigra National Park are located at a distance of less than 30 km from rural settlements, their use by hunting groups is illegal. To prevent further harm to the group of tigers studied, as well as to fulfill the obligations of the Russian Federation in preserving and increasing the population of this species, it is necessary to create extensive buffer zones around protected areas with a ban on hunting.
Список литературы
Atlas of the Hunter and Fisherman of the Primorsky Territory, 2004. Vladivostok: Besan. 181 p. [in Russian].
Barlow A.C.D., McDougal S., Smith J.L.D., Gurung B., Bhatta S.R., Kumal S., Mahato B., Tamang D.B., 2009. Temporal variation in tiger (Panthera tigris) population and its implication for monitoring // J. Mammal. V. 90. P. 472–478.
Bigon M., Harper J., Townsend K., 1989. Ecology: Individuals, Populations and Communities. M.: Mir. 667 p. [in Russian].
Chernova N.M., Bylova A.M., 1981. Ecology. Moscow: Prosveshcheniye. 255 p. [in Russian].
Global Tiger Recovery Program, 2010–2022 (St. Petersburg Declaration on Tiger Conservation at the International Tiger Forum (“Tiger Summit”), held in St. Petersburg, Russia, on November 21–24, 2010), 2010. Washington: Global Tiger Initiative Secretariat. 59 p.
Goodrich J.M., Kerley L.L., Smirnov E.N., Miquelle D.G., McDonald L., Quigley H.B., Hornocker M.G., McDonald T., 2008. Survival rates and causes of mortality of Amur tigers on and near Sikhote-Alin Biosphere Zapovednik // J. Zool. V. 276. P. 323–329.
Goodrich J.M., Miquelle D.G., Smirnov E.N., Kerley L.L., Quigley H.B., Hornocker M.G., 2010. Spatial structure of Amur (Siberian) tigers (Panthera tigris altaica) on Sikhote-Alin Biosphere Zapovednik, Russia // J. Mammal. V. 91. P. 737–748.
Karanth K.U., Nichols J.D., 1998. Estimating tiger densities in India from camera trap data using photographic captures and recaptures // Ecology. V. 79. P. 2852–2862.
Karanth K.U. and Nichols J.D., 2002. Monitoring tigers and their prey: A manual for researcher, managers and conservationists in tropical Asia. Center for wildlife studies, India. P. 193.
Kerley L.L., Borisenko M.M., 2017. Survival of the Amur tigers and their movement between the Lazovsky Reserve and Zov Tigra National Park // XII Far Eastern Conference of Nature Conservation Problems: Materials of the Scientific Conference in Birobidzhan, October 10–13, 2017, edited by E.Ya. Frisman. Birobidzhan: ICARP FEB RAS. P. 87–89. [in Russian].
Khalafyan A.A., 2007. STATISTICA 6. Statistical data analysis. M.: Binom-Press. 512 p. [in Russian].
Kolchin S.A., Soutyrina S.V., 2012. Mark trees and indirect communication between bears (Ursus arctos, Ursus thibetanus) and tigers (Panthera tigris) in the Sikhote-Alin // Bulletin of Game Biology. V. 9 (1). P. 5–16. [in Russian].
Lukarevskiy V.S., Lukarevskiy S.V., Kolchin S.A., Oleynikov A.Yu., 2021. Population structure and spatial distribution of the tiger (Panthera tigris, Felidae, Carnivora) in Southwestern Primorye (Russian Far East) // Ecologica Montenegrina. V. 43. P. 1–15.
Matiukhina D.S., Vitkalova A.V., Rybin A.N., Aramilev V.V., Shevtsova E.I., Miquelle D.G., 2016. Camera-trap monitoring of Amur tiger (Panthera tigris altaica) in Southwest Primorsky Krai // Nature Conservation Research. V. 1. Is. 3. P. 36–43.
McDougal S., 1977. The face of the tiger. London: Rivington Books. 180 p.
Nikolaev I.G., Yudin V.G., 1993. Tiger and man in conflict situations // Bulletin of the Moscow Society of Naturalists. V. 98. Is. 3. P. 23–36 [in Russian].
Riley M., Soutyrina S., Miquelle D., Hayward G., Goodrich J., Buskirk S., 2017. Comparison of methods for estimating Amur tiger abundance // Wildlife Biology: wlb.00253
Salkina G.P., 1993. The Current state of the tiger population in south Sikhote-Alin // Bulletin of the Moscow Society of Naturalists. V. 98. Is. 3. P. 45–53. [in Russian].
Salkina G.P., 2010. Poaching as the main factor in the mortality of the Amur tiger // The Amur tiger in Northeast Asia: Conservation Problems in the XXI Century: Intern. Conf., March 15–18, 2010, Vladivostok. Vladivostok: Dalnauka. P. 143–146.
Salkina G., 2011. The Tiger and its relations with other species in South Sikhote-Alin. Germany: Lambert Academic Publishing. 161 p.
Salkina G.P., 2013. Problems of conservation of the tiger in the Lazo Nature Reserve // Environmental Protection and Nature Management. V. 1. P.73–75. [in Russian].
Salkina G.P., Kolesnikov V.S, 2010. Changes in the number of tigers in the Lazovsky Reserve in 2005–2010 // The status of specially protected areas of the Far East (Materials of the conference dedicated to the 75th anniversary of the Lazovsky Reserve, Lazo, September 28–29, 2010). 2010. Vladivostok: Russkiy Ostrov. P. 186–191. [in Russian].
Salkina G.P., Kolesnikov V.S., Eryomin D.Yu., 2018. Population dynamics of the Amur tiger and the ungulates in Lazovsky Zapovednik // Modern Science: actual problems of theory and practice. Natural and technical sciences. V. 7. P. 25–34. [in Russian].
Salkina G.P., Kolesnikov V.S., Goryushin Yu.A., Pasynkov O.I., 2017. Experience of tiger photo census in the Lazovsky Reserve // XII Far Eastern Conference of Nature Conservation Problems: Materials of the Scientific Conference in Birobidzhan, October 10–13, 2017, edited by E.Ya. Frisman. Birobidzhan: ICARP FEB RAS. P. 109– 111. [in Russian].
Salkina G.P., Poddubnaya N.Ya., Kolesnikov V.S., Kolchin S.A., 2019. Turnover of individuals in the tiger population (Panthera tigris L., 1758) of the Lazovski Reserve and Zov Tiger National Park // Ecology and Evolution: New Challenges: Proceedings of the International Symposium dedicated to the celebration of 100th anniversary of RAS Academician S.S. Shwartz (April 1–5, 2019, Ekaterinburg, Russia). Ekaterinburg: Liberal Arts University – University for Humanities. P. 91–94. [in Russian].
Salkina G.P., Solomkina N.V., 2005. Identification of Amur tigers using dogs // State of Specially Protected Areas (Materials of the conference dedicated to the 70th anniversary of the Lazovsky Reserve, Lazo, April 19–20, 2005). Vladivostok: Russkiy Ostrov. P. 147–150. [in Russian].
Skidmore A., 2021. Using crime script analysis to elucidate the details of Amur tiger poaching in the Russian Far East // Crime Sci. V. 10 (16). P. 1–25.
Smith J.L.D., 1993. The role of dispersal in structuring the Chitwan tiger population // Behaviour. V. 124. P. 165–195.
Smith J.L.D., McDougal C., Sunquist M.E., 1987. Female land tenure system in tigers // Tigers of the World: The Biology, Biopolitics, Management and Conservation of an Endangered and Species, ed. R.L. Tilson, U.S. Seal. New Jersey: Noyes Publication. P. 97–109.
Soutyrina S.V., Riley M.D., Goodrich D.M., Seryodkin I.V., 2013. A population estimate of Amur tigers using camera traps. Vladivostok: Dalnauka. 156 p.
Soutyrina S.V., Seryodkin I.V., Mikell D.G., 2014. Change in the composition of the Amur tiger group in the Sikhote-Alin Reserve according to photo records // Izvestia of Samara Scientific Center of the Russian Academy of Sciences. V. 16. № 1(4). P. 1176–1179. [in Russian].
Statsoft.Statistica, 2010 edition. Kernel release 5.5 A. https://www.statistica.com
Yudin V.G., Yudina E.V., 2009. The Tiger of the Russian Far East. Vladivostok: Dalnauka. 485 p. [in Russian].
Yudin V.G., Yudina E.V., 2009. The Tiger of the Russian Far East. Vladivostok: Dalnauka. 485 p. [in Russian].
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал