Успехи современной биологии, 2023, T. 143, № 6, стр. 553-564
Современная концепция гормезиса: обзор проблемы и значение для экологии
Е. А. Ерофеева 1, *, Д. Б. Гелашвили 1, Г. С. Розенберг 2
1 Нижегородский государственный университет им. Н.И. Лобачевского
Нижний Новгород, Россия
2 Институт экологии Волжского бассейна РАН – филиал Самарского федерального
исследовательского центра РАН
Тольятти, Россия
* E-mail: ele77785674@yandex.ru
Поступила в редакцию 25.06.2023
После доработки 01.08.2023
Принята к публикации 02.08.2023
- EDN: ZIBHKR
- DOI: 10.31857/S0042132423060030
Аннотация
В настоящее время установлено, что при воздействии на живые организмы различных экологических факторов (природных – абиотических и биотических, а также антропогенных) гормезис представляет собой достаточно распространенное явление. Гормезис обнаружен у разных групп организмов и практически на всех уровнях организации живых систем от клетки до экосистемы. В то же время всесторонний анализ значения гормезиса для экологии не проводился. В данном обзоре рассматривается современная концепция гормезиса, представленная в зарубежной литературе, а также ее значение для различных разделов экологии.
ВВЕДЕНИЕ
Долгое время гормезис рассматривался как редкое и недостаточно понятное явление и фактически не учитывался при изучении реакций живых систем разного уровня организации на различные факторы среды (Calabrese, 2008; Agathokleous, Calabrese, 2020). В последние годы отмечается значительный интерес к этому явлению за рубежом, поскольку многочисленными исследованиями продемонстрировано, что горметические ответы являются достаточно распространенными (Calabrese, Blain, 2009, 2011; Agathokleous, Calabrese, 2020; Shahid et al., 2020; Jalal et al., 2021). Явление гормезиса обнаружено на различных уровнях организации живых систем от клетки (Zhang et al., 2017; Gopi, Rattan, 2019) и организма (Calabrese, 2008; Agathokleous, Calabrese, 2020) до популяций (Sial et al., 2018; Tang et al., 2019), сообществ (Agathokleous, Calabrese, 2020; Fan et al., 2020; Agathokleous et al., 2021a) и даже экосистем (Agathokleous, 2018; Erofeeva, 2023). Горметические ответы возникают у эволюционно различных групп организмов (Agathokleous, Calabrese, 2020): бактерий (Cui et al., 2018; Wang et al., 2021), грибов (Di et al., 2016; Cong et al., 2018), растений (Calabrese, Blain, 2009; Agathokleous et al., 2020a), животных, включая человека (Calabrese, Blain, 2011; Berry, López-Martínez, 2020). Все это указывает на то, что концепция гормезиса касается не только различных сфер токсикологии, медицины и биологии, а также многих важнейших фундаментальных и прикладных вопросов в области экологии. В последние годы зарубежными авторами опубликован ряд обзорных статей, затрагивающих отдельные экологические аспекты явления гормезиса (Costantini et al., 2010; Agathokleous, 2018; Agathokleous, Calabrese, 2020; Agathokleous et al., 2021a). Однако всестороннего анализа значения современной концепции гормезиса для экологии не проводилось. В данном обзоре рассмотрена современная концепция гормезиса, проведен анализ ее значения для различных разделов экологии в фундаментальном и прикладном аспектах, а также оценены перспективы использования данной концепции в экологии.
Следует отметить, что данный обзор сосредоточен на концепции гормезиса, принятой в настоящее время за рубежом, так как это направление очень быстро развивается и отличается по ряду положений от точки зрения, транслируемой в российских научных источниках. Несомненно, отечественные исследования радиационного и химического гормезиса имеют существенное значение для развития представлений о горметических ответах организмов. Однако для анализа всего материала по гормезису, существующего в отечественной и зарубежной литературе, требуется формат монографии. Поэтому анализ отечественных исследований остался за рамками данного обзора, цель которого – знакомство читателей с концепцией гормезиса, развиваемой зарубежными исследователями.
СОВРЕМЕННАЯ КОНЦЕПЦИЯ ГОРМЕЗИСА
В настоящее время в зарубежной литературе термином гормезис (от греч. ορμεσις – быстрое движение, стремление) обозначают двухфазный адаптационный ответ живой системы на стрессовый фактор (или факторы) среды, при котором низкие дозы фактора оказывают стимулирующее воздействие, а высокие дозы фактора вызывают ингибирующий эффект (Calabrese, 2008; Agathokleous, Calabrese, 2020).
Следует отметить, что при хроническом воздействии данной дозы/концентрации стрессора горметическая стимуляция возникает на начальных этапах его воздействия. При длительном воздействии может наблюдаться вторая фаза гормезиса – ингибирование. Например, показано, что хроническое воздействие низких доз различных поллютантов (хром, кадмий, ДДТ) на растения (Jia et al., 2013; Mitton et al., 2014) сначала вызывает стимулирующий горметический эффект в отношении перекисного гомеостаза растений (антиоксидантное действие поллютантов), а при продолжительном воздействии переходит в ингибирующий эффект (прооксидантное действие поллютантов).
Таким образом, в отличие от классических зависимостей доза–эффект (пороговых и непороговых), используемых в токсикологии, экотоксикологии, радиоэкологии, горметическая кривая доза–эффект не монотонна, то есть имеет экстремум, поскольку направление ответа живой системы отличается в диапазоне низких и высоких доз фактора (Calabrese, 2008).
Выделяют два вида горметических кривых (рис. 1) (Calabrese, 2008; Calabrese, Blain, 2009):
Рис. 1.
Два вида горметических кривых доза–эффект (по: Calabrese, Blain, 2009, с изменениями): (а) – инвертированная U-образная кривая – стимулирующий эффект низких доз и ингибирующий эффект высоких доз; (б) – U-образная кривая – снижение повреждающего эффекта низкими дозами и увеличение повреждающего эффекта высокими дозами. Например, низкие концентрации тяжелых металлов снижают интенсивность перекисного окисления липидов в клетках растений, а высокие концентрации данных поллютантов стимулируют этот процесс.
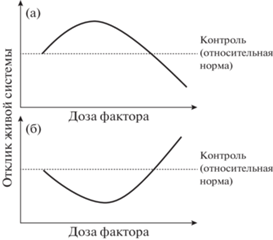
1) Инвертированная U-образная кривая (inverted U-shaped curve) или инвертированная J-образная кривая (inverted J-shaped curve), встречающаяся наиболее часто и представляющая стимуляцию низкими дозами фактора и ингибирование высокими дозами (рис. 1а).
2) U-образная кривая (U-shaped curve) или J-образная кривая (J-shaped curve), представляющая снижение повреждающего эффекта в области низких доз и его увеличение при высоких дозах фактора (рис. 1б).
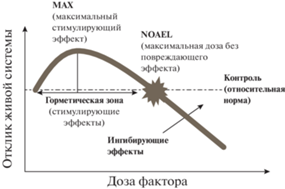
В пределах горметической кривой выделяют универсальные количественные характеристики, свойственные горметическим ответам любых организмов (Calabrese, 2008; Agathokleous, Calabrese, 2020) (рис. 2). Горметическая зона кривой представляет собой диапазон доз фактора, обладающих стимулирующим эффектом относительно контрольного уровня (условной нормы). Эта зона характеризуется максимальным стимулирующим эффектом MAX (выражается в % от контроля и чаще всего составляет 130–160%, иногда до 200%) и шириной диапазона стимулирующих доз (обычно не более двух порядков, но в 2% случаев превышает три порядка). Кроме того, выделяют максимальную дозу, не вызывающую повреждающего (ингибирующего) эффекта NOAEL (no-observed adverse effect level) (Calabrese, 2008; Calabrese, Blain, 2009; Agathokleous, Calabrese, 2020).
Рис. 2.
Количественные характеристики горметической кривой доза–эффект. NOAEL (no-observed adverse effect level) – максимальная доза, не оказывающая повреждающего (ингибирующего) эффекта. Горметическая зона – диапазон доз, вызывающих стимулирующий эффект относительно контрольного уровня. Эта зона характеризуется шириной стимулирующего диапазона и максимальным стимулирующим эффектом (MAX) (по: Agathokleous, Calabrese, 2020, с изменениями).
Полагают, что биологический смысл стимулирующего эффекта низких доз стресс-фактора в горметической зоне – это сверхкомпенсация параметров живой системы, которая необходима для повышения устойчивости к возможному последующему воздействию сильного стресс-фактора (факторов) (Calabrese, 2008; Agathokleous, Calabrese, 2020). При сверхкомпенсации живая система не только компенсирует нарушения, вызванные низкодозовым стрессором, но и повышает эффективность функционирования и активность защитных систем. Например, у растений гербициды в низких концентрациях стимулируют рост, фотосинтез, активность антиоксидантной защиты (Jalal et al., 2021; Costa et al., 2023) и даже урожайность (Pincelli-Souza et al., 2020).
Показано, что низкие дозы различных стрессовых факторов (природных – абиотических и биотических, а также антропогенных), вызывающих горметическую сверхкомпенсацию повышают устойчивость к сильным стрессорам. Это явление было названо прекондиционированием (preconditioning – подготовка, предобработка), в качестве синонима которого также используется термин прайминг (priming – грунтовка, в смысле предварительной обработки) (Martinez-Medina et al., 2016; Calabrese, 2016a; Agathokleous et al., 2020a). Путем прекондиционирования гормезис может повышать резистентность к высоким дозам стрессового фактора, низкие дозы которого вызывают горметическую стимуляцию, или даже к другим сильным стрессорам. В последнем случае наблюдается эффект, называемый кросс-адаптацией или кросс-толерантностью (Walter et al., 2013; Foyer et al., 2016), то есть повышение устойчивости организма к сильному стрессору в результате предшествующего воздействия умеренного стрессора иной природы. Примером эффекта кросс-адаптации может служить воздействие на мух Drosophila melanogaster мертвых спор энтомопатогенного гриба Metarhizium robertsii, стимулирующее их фертильность, увеличивающее продолжительность жизни, повышающее устойчивость к высоким температурам (McClure et al., 2014).
Таким образом, за счет горметического прекондиционирования живая система прогнозирует изменения среды и может значительно повысить эффективность поддержания гомеостаза в нестабильной среде (Calabrese, 2008; Agathokleous, Calabrese, 2020).
Кроме того, обнаружено явление посткондиционирования (postconditioning – постобработка), возникающее при воздействии низких горметических (стимулирующих) доз фактора на живую систему после сильного стрессора. В случае посткондиционирования горметические дозы повышают эффективность восстановления организма или клеток, подвергнутых повреждающему воздействию сильного стресс-фактора (Wiegant et al., 2011; Calabrese, 2016a). При этом дозы фактора, наиболее эффективно вызывающие прекондиционирование, обладают также наиболее выраженным эффектом посткондиционирования (Calabrese, 2016b).
В пределах горметической зоны может наблюдаться явление горметического компромисса (hormetic trade-off), когда горметическая сверхкомпенсация отмечается только для некоторых показателей живой системы, что обусловлено ограниченностью ресурсов системы (Agathokleous, Calabrese, 2020). Вероятно, в первую очередь сверхкомпенсируются параметры, наиболее актуальные для прогнозируемой встречи с сильным стрессором (стрессорами) (Agathokleous, Calabrese, 2020).
Молекулярный механизм гормезиса до сих пор остается неясным. Существуют многочисленные гипотезы, которые описывают молекулярные процессы на уровне клетки и организма, приводящие к гормезису (Calabrese, 2013). В частности, предполагается, что агент в стимулирующих и ингибирующих дозах воздействует на различные типы/подтипы рецепторов клетки или разные клеточные сигнальные пути, что индуцирует немонотонный двухфазный паттерн зависимости доза–эффект (Calabrese, 2013).
Считается, что не существует единого молекулярного механизма гормезиса (например, рецептора или клеточного сигнального пути) даже у субпопуляций одного вида. Горметическая стимуляция может достигаться за счет различных клеточных процессов в зависимости от вида организмов, генотипа, индуцирующего агента (Agathokleous et al., 2020a). При этом на уровне организма происходит умеренная активация защитных систем (стрессовые гормоны, антиоксидантная защита, стрессовые белки и т.д.) (Calabrese, 2013).
Поскольку поддержание гомеостаза на уровне клетки, организма, популяции, экосистемы происходит путем качественно различных регуляторных механизмов (Шилов, 2019), то механизмы горметической стимуляции также имеют качественные особенности на этих уровнях. Тем не менее, это единая система, поэтому гормезис на уровне организма у значительного количества особей в популяциях может влиять на популяционные характеристики. Изменение состояния популяции в свою очередь влияет на функции вида в сообществе, далее – на роль сообщества в обеспечении потока энергии и круговорота веществ экосистемой, то есть имеет значение для гомеостаза и, соответственно, устойчивости экосистем. Таким образом, гормезис на более низких уровнях, надо полагать, является основой горметического ответа на более высоких уровнях организации живых систем.
ГОРМЕЗИС НА УРОВНЕ ОРГАНИЗМА
Как известно, эффекты различных экологических факторов на уровне организма рассматривает факториальная экология или аутэкология. В настоящее время горметические ответы на разнообразные экологические стрессоры наиболее изучены на организменном уровне живых систем.
Явление гормезиса обнаружено у различных групп организмов (растений: высших растений и водорослей; животных: позвоночных и беспозвоночных животных, бактерий, архей, грибов, простейших) (табл. 1). При этом у многих групп гормезис показан для абиотических, биотических и разнообразных антропогенных факторов. В табл. 1 представлены только некоторые примеры факторов и групп организмов, демонстрирующих гормезис, полученные при анализе литературы за последние годы. Фактически доказательства гормезиса у разных организмов намного обширнее, что проанализировано статистически в ряде обзорных статей (Calabrese, Baldwin, 1999; Calabrese, Blain, 2005, 2009, 2011).
Таблица 1.
Примеры экологических факторов, вызывающих гормезис у разных групп организмов
| Группы организмов | Экологические факторы | Источники | ||
|---|---|---|---|---|
| природные | ||||
| абиотические | биотические | антропогенные | ||
| Бактерии, археи | pH среды, температура | Терпены растений | Тяжелые металлы, ионизирующая радиация, антибиотики | Kudryasheva, Rozhko, 2015; Martínez, 2017; Xu et al., 2020; Schirrmacher, 2021 |
| Простейшие (инфузории) | – | – | Ионизирующее и неионизирующее излучение, наночастицы меди | Mortimer et al., 2010; Obodovskiy, 2019 |
| Грибы (дрожжи, базидиомицеты, фитопатогенные грибы) | – | – | Фунгициды, тяжелые металлы | Calabrese, Baldwin, 1999; Zied et al., 2017; Morkunas et al., 2018; Zhang et al., 2019; Cong et al., 2018; Agathokleous, Calabrese, 2021 |
| Растения (высшие растения, водоросли) | Макро- и микроэлементы, температура почвы и воздуха, влажность почвы, интенсивность света и его спектр | Элиситоры, аллело- химические вещества |
Ионизирующее и неионизирующее излучение, тяжелые и редкоземельные металлы, наночастицы тяжелых металлов, антибиотики, пестициды, углеводороды, формальдегид, пластики и микропластики, приземный озон | Calabrese, Blain, 2009; Xu et al., 2012; Erofeeva, 2013, 2018, 2021, 2022; Motai et al., 2017; Chen et al., 2018; Agathokleous et al., 2018, 2019a, 2019b, 2019c, 2020a, 2020b, 2021b; Shahid et al., 2020; Jalal et al., 2021; Calabrese, Agathokleous, 2021 |
| Животные (круглые и кольчатые черви, ракообразные, моллюски, насекомые, рыбы, амфибии, рептилии, птицы, млекопитающие) | Гипоксия, температура, обезвоживание, гравитация | Избыточная плотность популяции, дефицит пищевых ресурсов (умеренное голодание) | Ионизирующее и неионизирующее излучение, пестициды, инсектициды, тяжелые металлы, фармпрепараты, полихлорированные бифенилы, наночастицы | Roberts et al., 2007; Diaz et al., 2008; Calabrese, Blain, 2009; Drobne et al., 2009; Hashmi et al., 2015; Moore et al., 2015; Nielsen, Roslev, 2018; Cao et al., 2019; Deng et al., 2019; Vaiserman et al., 2021; Berry, López-Martínez, 2020; Schirrmacher, 2021 |
Гормезис обнаружен и при комбинированном воздействии нескольких факторов среды. Так, например, показаны горметические ответы у водного растения Myriophyllum aquaticum при воздействии антибиотиков и тяжелых металлов одновременно (Guo et al., 2020), у наземных растений – при обработке бинарными смесями гербицидов (Belz, Piepho, 2017), у фитопатогенных грибов – при воздействии бинарных смесей фунгицидов (Zhang et al., 2019). Однако закономерности горметических ответов при совместном воздействии различных факторов среды (биотических, абиотических, антропогенных) до сих пор остаются мало изученными, хотя именно этот тип воздействия чаще всего наблюдается в реальных условиях (Agathokleous et al., 2020a).
Возможно, гормезис может быть одним из механизмов, лежащих в основе закона совокупного действия факторов (закон Митчерлиха–Бауле), утверждающего, что величина урожая зависит не только от какого-нибудь одного (пусть даже лимитирующего) фактора, но и от всей совокупности действующих факторов одновременно (Harmsen, 2000). Слабые стресс-факторы могут вызывать гормезис, который через эффекты прекондиционирования и посткондиционирования влияет на устойчивость организмов к сильным стрессовым воздействиям. Например, низкие концентрации никеля вызывают горметическую стимуляцию роста корневой системы у Arabidopsis thaliana (L.) Heynh. и повышают устойчивость к засолению (эффект прекондиционирования), вызванному высокими концентрациям хлорида натрия (Rahavi et al., 2011). Аналогично, у ячменя Hordeum vulgare L. низкие дозы озона и умеренный дефицит воды повышают устойчивость к высоким концентрациям меди. Кроме того, умеренная засуха также положительно влияет на резистентность ячменя к кадмию (Kacienė et al., 2017).
Краеугольным камнем факториальной экологии является кривая Шелфорда (рис. 3), которая графически описывает закон толерантности Шелфорда. Показано, что если использовать классификацию зон кривой: зона оптимума и две зоны стресса (Shelford, 1913), применяемую и в настоящее время за рубежом (Helaouёt, Beaugrand, 2009; Hatfield, Prueger, 2015), то гормезис возникает в зоне стресса. При умеренном отклонении экологического фактора от оптимума наблюдается горметическая стимуляция, а при значительном отклонении от оптимума – ингибирование (рис. 3) (Erofeeva, 2021). Также продемонстрировано, что гормезис через эффект прекондиционирования может влиять на границы толерантности к данному экологическому фактору и даже к другим экологическим факторам за счет горметической кросс-адаптации, делая эти границы более лабильными, что особенно важно для лимитирующих факторов ареала (Erofeeva, 2021). Гормезис как проявление биологической пластичности (способности генотипа продуцировать различные фенотипы при адаптации к среде) (Agathokleous et al., 2019d) также подвержен процессу эволюции, то есть способность к горметической сверхкомпенсации параметров отличается у разных видов. Предполагается, что наиболее высокой способностью к гормезису обладают эврибионтные виды, имеющие широкий диапазон толерантности ко многим факторам среды (Erofeeva, 2021). Таким образом, включение концепции гормезиса в анализ закономерностей воздействия экологических факторов на живые системы имеет большое значение для развития факториальной экологии в рамках современных представлений об адаптивных реакциях живых систем.
ГОРМЕЗИС НА УРОВНЕ ПОПУЛЯЦИЙ
Статические (численность, плотность и др.) и динамические показатели (рождаемость, смертность и др.) состояния популяций также могут иметь двухфазные горметические изменения. Стимулирующие эффекты низкодозовых стрессоров в отношении индикаторов состояния популяций указывают на проявление гормезиса на популяционном уровне, основой которого, несомненно, является горметическая стимуляция на уровне организма, то есть особей популяции. Например, низкие концентрации остаточного Al(III) увеличивают за счет эффекта гормезиса рост численности бактерий в 3.7 раза, относительно контроля в бактериальной пленке водоочистных сооружений, а высокие концентрации Al(III) снижают этот показатель (Cui et al., 2018). Вспышки численности вредителей сельскохозяйственных культур (насекомых, клещей), вызванные низкими дозами пестицидов, обусловлены гормезисом (Morse, 1998; Tang et al., 2019). Например, у тли Myzus persicae Sulzer инсектициды неоникотиноиды вызывают в низких дозах улучшение демографических показателей популяции, а также повышают плодовитость, что сопровождается увеличением экспрессии генов детоксикации инсектицидов (Sial et al., 2018). Пиретроидный инсектицид дельтаметрин вызывает у кукурузного долгоносика Sitophilus zeamais, Motschulsky двухфазные зависимости доза–эффект для чистого коэффициента воспроизводства популяции, внутренней скорости популяционного роста и прогнозируемой численности популяции. Таким образом, низкие концентрации инсектицида повышают воспроизводство популяции относительно контрольного уровня (Guedes et al., 2010).
Следует отметить, что разные субпопуляционные группы могут проявлять разную способность к гормезису. Так, продемонстрировано (Belz et al., 2018), что быстрорастущие и медленнорастущие растения салата Lactuca sativa имели разную способность к гормезису при воздействии шести гербицидов. Горметическая стимуляция роста низкими дозами гербицидов отмечалась только у быстрорастущих субпопуляций, но не у медленнорастущих. Поэтому в целом в популяции горметический эффект не выявлялся. Таким образом, гормезис возникает не только на уровне организма, но и на популяционно-видовом уровне.
Полагают, что гормезис может влиять на эволюцию популяций через усиление конкурентоспособности, устойчивости к стрессовым факторам среды у особей и, соответственно, воспроизводства ими потомства. Величина стимулирующего эффекта гормезиса у чувствительных к воздействию низких доз стресс-факторов и устойчивых генотипов может отличаться на несколько порядков, что значительно влияет на эффективность их размножения, создает давление отбора и эволюционные паттерны в популяциях (Schreck, 2010; Belz, 2018; Agathokleous, Calabrese, 2020).
Таким образом, явление гормезиса имеет существенное значение для генетической структуры и приспособленности популяций и, возможно, является одним из механизмов как эволюции, так и подержания гомеостаза в популяциях, повышая их устойчивость к стресс-факторам среды. Изучение гормезиса в рамках популяционной экологии позволяет существенно продвинуться в понимании закономерностей взаимодействия популяций со средой.
ГОРМЕЗИС НА УРОВНЕ СООБЩЕСТВ И ЭКОСИСТЕМ
Гормезис в сообществах и экосистемах наименее изучен, по сравнению с популяционно-видовым и организменным уровнями организации живых систем (Costantini et al., 2010; Agathokleous, 2018). Тем не менее существуют доказательства этого явления на уровне сообществ для показателей структуры и функционирования. Например, обнаружена горметическая стимуляция низкими дозами поллютантов (толуол, тяжелые металлы, гербициды) показателей, отражающих способность сообществ почвенных микроорганизмов осуществлять биохимические процессы в почве (активность щелочной фосфатазы, почвенное дыхание) (Fan et al., 2020). В исследовании (Han et al., 2019) установлено, что горметические (стимулирующие) концентрации кадмия, увеличивающие активность почвенной щелочной фосфатазы, вызывают изменение состава сообщества почвенных микроорганизмов путем стимуляции размножения бактерий (увеличение относительного обилия), выделяющих в почву этот фермент. Продемонстрировано (Wang et al., 2021), что кадмий в низких дозах увеличивает видовое разнообразие сообщества почвенных бактерий и грибов. Стимулирующий горметический эффект обнаружен и при комбинированном воздействии поллютантов (кадмия и свинца) на обилие почвенных бактерий и грибов (Fan et al., 2021).
Помимо выявления воздействия тяжелых металлов, в последние годы появляется все больше исследований, демонстрирующих стимулирующие эффекты низких доз микропластиков (частицы любых видов пластиков длиной менее 5 мм) в отношении бактериальных сообществ (Agathokleous et al., 2021a). Так, наночастицы полистерола (<1 мкм) увеличивают относительное обилие видов в сообществе бактерий, обитающих в водах Арктики (Agathokleous, 2018). Мембранный полиэтилен и волокнистый полипропилен повышают альфа-разнообразие почвенной микробиоты (Yi et al., 2021). Также микропластики увеличивают альфа-разнообразие кишечного микробиома у рыб (Gu et al., 2020) и мышей (Li et al., 2020). Как известно, пластики содержат, кроме химически инертных соединений, токсичные вещества, которые выделяются в окружающую среду. Среди них наиболее токсичными считаются бисфенолы A, B, F, S. В частности, бисфенол А – агонист рецепторов эстрогена, в больших дозах нарушающий функционирование эндокринной системы животных и человека (Chouhan et al., 2014; Lo et al., 2021). Однако в низких концентрациях токсиканты пластиков оказывают горметические эффекты аналогично другим поллютантам.
Горметические эффекты низких доз факторов среды обнаружены и для фитоценозов. Например, в лесных сообществах осаждение азота в почву (15 и 35 кг N га–1 год–1) стимулирует секвестрацию CO2 (De Vries et al., 2014), то есть углеродный цикл. Низкие уровни осаждения азота (8.7 и 13.4 кг N га–1 год–1 для растительности с открытым и закрытым пологом соответственно) увеличивают видовое богатство сообществ травянистых растений более чем 15 000 лесных, луговых, кустарниковых и лесных участков по всей континентальной части Соединенных Штатов, а более высокие концентрации азота снижают этот показатель (Simkin et al., 2016). Данные факты рассматриваются как проявление гормезиса на уровне фитоценозов (Agathokleous, 2018).
Следует отметить, что пока ценотические эффекты гормезиса обнаружены только для некоторых типов сообществ, что обусловлено незначительным количеством целенаправленных исследований этого вопроса.
Кроме того, ряд метаанализов показали возможность гормезиса на уровне экосистем на примере стимуляции первичной продукции экосистем при длительном изменении климатических факторов (повышение температуры, количества осадков, концентрации CO2) в полевых экспериментах (Smith et al., 2000; Rustad et al., 2001; Dormann, Woodin, 2002; Wu et al., 2011). Например, в метаанализе (Wu et al., 2011), основанном на данных 85 многолетних полевых экспериментов, выполненных в различных биомах (бореальный лес, влажный тропический лес, лесостепь и др.), показано, что экспериментальное потепление и увеличение количества осадков стимулирует надземную биомассу (в среднем на 27 и 12% соответственно) древесных растений и потоки углерода в экосистеме, а также увеличивает суммарную первичную продукцию экосистемы, фотосинтез экосистемы, экосистемное дыхание и чистый экосистемный обмен. А снижение температуры и количества осадков в полевых экспериментах оказывает ингибирующий эффект на указанные показатели. Данные стимулирующие эффекты температуры и количества осадков можно рассматривать как гормезис, поскольку в указанных экосистемах, например во влажном тропическом лесу, не наблюдается дефицита данных факторов. Тем не менее гормезис на уровне экосистем требует дальнейшего изучения и более обширных доказательств.
Приведенные выше факты указывают, что гормезис через изменение структуры и функций сообществ, по-видимому, может влиять на способность экосистемы обеспечивать биогенный круговорот веществ и потоки энергии и, следовательно, имеет значение для устойчивости экосистемы, а также, возможно, процессов сукцессии как формы функциональных адаптаций на экосистемном уровне. Поскольку на уровне биосферы различные экосистемы образуют общую систему, осуществляющую биогеохимические циклы (Шилов, 2019), то влияние гормезиса на их состояние может иметь глобальные последствия на уровне биосферы.
Вышеуказанные аспекты еще только предстоит подробно изучить. Тем не менее можно констатировать, что решение этих вопросов сыграет серьезную роль в развитии синэкологии и даже биосферологии.
ЗНАЧЕНИЕ ГОРМЕЗИСА ДЛЯ ОЦЕНКИ КАЧЕСТВА СРЕДЫ, ЭКОЛОГИЧЕСКИХ РИСКОВ И НОРМИРОВАНИЯ АНТРОПОГЕННОЙ НАГРУЗКИ
Определение зависимостей доза–эффект для реакций живых организмов находит широкое применение в оценке качества окружающей среды (биоиндикация, биотестирование, экологический мониторинг), в нормировании антропогенной нагрузки, в оценке экологических рисков (Гелашвили и др., 2016). В настоящее время для данных целей используются линейные и пороговые модели доза–эффект, являющиеся монотонными, то есть не имеющими экстремумов (Calabrese, 2008). До сих пор горметическая модель доза–ответ не включена в нормативные документы и методики как в нашей стране (Гелашвили и др., 2016), так и за рубежом (Agathokleous, Calabrese, 2020), хотя вероятность такого двухфазного ответа живых систем на антропогенное воздействие достаточно высока (Agathokleous et al., 2021c).
Игнорирование гормезиса может приводить к неадекватному нормированию техногенной нагрузки, а также к некорректным оценкам экологической ситуации и прогнозам ее развития. Например, даже значительный уровень загрязняющих веществ в окружающей среде может не оказывать ингибирующего эффекта на виды-биоиндикаторы, которые предварительно испытывали воздействие низких доз поллютантов и за счет горметической стимуляции приобрели повышенную устойчивость к загрязнению (явление горметического прекондиционирования). В итоге это приведет к заниженной оценке уровня антропогенной нагрузки при анализе качества среды с помощью методов биоиндикации. Аналогичная ситуация наблюдается, когда нормативы антропогенного воздействия не учитывают явления гормезиса. Низкие дозы поллютантов, не превышающие нормативы, могут вызывать стимулирующий горметический эффект. Данный эффект в случае кумуляции может стать отрицательным. Например, при длительном хроническом воздействии низких доз различных поллютантов (хром, кадмий, ДДТ) на растения (Jia et al., 2013; Mitton et al., 2014) показано, что стимулирующий горметический эффект в отношении перекисного гомеостаза растений (антиоксидантное действие поллютантов) переходил в ингибирующий эффект (прооксидантное действие поллютантов).
В последнее время за рубежом отмечаются некоторые сдвиги в плане использования горметической модели доза–эффект для оценки экологических рисков. В 2018 г. Агентство по охране окружающей среды США EPA (Environmental Protection Agency) опубликовало предложение, в рамках которого оно впервые в своей истории рассмотрело современные модели доза–ответ. Признавая значительные доказательства широкого распространения нелинейных реакций у организмов, агентство допустило использование горметической модели для оптимизации оценки рисков в области низких доз поллютантов, что было утверждено 6.01.2021 г. Ранее для оценки экологических рисков EPA, основываясь на убеждении, что любая доза поллютанта в какой-то степени вредна для организма, использовало только линейную непороговую модель LNT (linear-no-threshold), которая предполагает, что риск прямо пропорционален дозе/воздействию вплоть до нулевой дозы/воздействия (Agathokleous et al., 2021c).
Таким образом, использование горметической модели доза–эффект необходимо для повышения объективности методов и подходов, используемых для оценки качества среды, экологических рисков и нормирования антропогенной нагрузки.
ЗАКЛЮЧЕНИЕ
Представленный в данной работе анализ показал, что современная концепция гормезиса является актуальной для развития многих разделов экологии, рассматривающих различные уровни организации живого от организма до биосферы. Надо полагать, что это обусловлено системным значением гормезиса. Фактически, он представляет собой проявление общего свойства живых систем разного уровня организации прогнозировать (предвидеть) изменение среды и подготавливаться к этим изменениям путем сверхкомпенсации своих параметров, что актуально для выживания в изменчивой среде с постоянными воздействиями сильных стресс-факторов. Это позволяет живым системам эффективно поддерживать своей гомеостаз, несмотря на нестабильность среды. Таким образом, скорее всего, гормезис представляет собой принцип адаптации живых систем к изменчивой среде. Поиски универсального механизма гормезиса до сих пор не увенчались успехом. По-видимому, такого механизма просто не существует. Горметическая стимуляция параметров системы может реализовываться самыми различными путями в зависимости от уровня организации (клетка, организм, популяция, сообщество, экосистема, биосфера), от нарушений параметров системы, вызванных умеренным стрессовым воздействием (воздействиями), которое провоцирует горметическую сверхкомпенсацию параметров системы, от ресурсов системы и т.д. Явления горметического прекондиционирования и посткондиционирования пока обнаружены только на уровне организма. Гипотетически они также могут наблюдаться в популяциях, сообществах и экосистемах и, может быть, даже на уровне биосферы, поскольку именно за счет этих явлений реализуются адаптационные эффекты гормезиса.
Следует отметить, что горметические эффекты различных антропогенных факторов, в том числе поллютантов, не означают, что они “полезны”, поскольку стимуляция и ингибирование определяются дозой/концентрацией и продолжительностью воздействия.
Широкое распространение гормезиса обусловлено тем, что на уровне организма он основан на явлении стресса. Следует подчеркнуть, что еще создатель концепции стресса Селье (Selye, 1974) отмечал, что умеренные воздействия вызывают положительный стресс (эустресс), а чрезмерное напряжение организма приводит к негативному стрессу (дистрессу), который может вызвать истощение ресурсов организма и гибель. Горметические эффекты на уровне популяций, сообществ и экосистем показывают, что для данных биологических уровней также характерны универсальные адаптационные ответы, аналогичные стрессу на уровне организма. Однако их закономерности и качественные особенности на каждом биологическом уровне являются предметом будущих исследований.
В данном обзоре проанализированы и отмечены только наиболее важные вопросы, связанные со значением современной концепции гормезиса для экологии, с целью привлечения внимания специалистов к этой проблеме. Проведение детального изучения гормезиса в экологии, уточнение и развитие концепции экологического гормезиса еще только предстоят.
Список литературы
Гелашвили Д.Б., Безель В.С., Романова Е.Б. и др. Принципы и методы экологической токсикологии. Н.Н.: ННГУ, 2016. 702 с.
Шилов И.А. Экология. М.: Юрайт, 2019. 512 с.
Agathokleous E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety // Ecotoxicol. Environ. Saf. 2018. V. 148. P. 1042–1053. https://doi.org/10.1016/j.ecoenv.2017.12.003
Agathokleous E., Calabrese E.J. A global environmental health perspective and optimisation of stress // Sci. Total Environ. 2020. V. 704 (135263). https://doi.org/10.1016/j.scitotenv.2019.135263
Agathokleous E., Calabrese E.J. Fungicide-induced hormesis in phytopathogenic fungi: a critical determinant of successful agriculture and environmental sustainability // J. Agric. Food Chem. 2021. V. 69 (16). P. 4561–4563. https://doi.org/10.1021/acs.jafc.1c01824
Agathokleous E., Kitao M., Calabrese E.J. Human and veterinary antibiotics induce hormesis in plants: scientific and regulatory issues and an environmental perspective // Environ. Int. 2018. V. 120. P. 489–495. https://doi.org/10.1016/j.envint.2018.08.035
Agathokleous E., Belz R.G., Calatayud V. et al. Predicting the effect of ozone on vegetation via linear non-threshold (LNT), threshold and hormetic dose—response models // Sci. Total Environ. 2019a. V. 649. P. 61–74. https://doi.org/10.1016/j.scitotenv.2018.08.264
Agathokleous E., Feng Z.Z., Iavicoli I., Calabrese E.J. The two faces of nanomaterials: a quantification of hormesis in algae and plants // Environ. Int. 2019b. V. 131. P. 105044. https://doi.org/10.1016/j.envint.2019.105044
Agathokleous E., Kitao M., Harayama H., Calabrese E.J. Temperature-induced hormesis in plants // J. Forest. Res. 2019c. V. 30. P. 13–20. https://doi.org/10.1007/s11676-018-0790-7
Agathokleous E., Kitao M., Calabrese E.J. Hormesis: a compelling platform for sophisticated plant science // Trends Plant Sci. 2019d. V. 24 (4). P. 318–327. https://doi.org/10.1016/j.tplants.2019.01.004
Agathokleous E., Kitao M., Calabrese E.J. Hormesis: highly generalizable and beyond laboratory // Trends Plant Sci. 2020a. V. 25 (11). P. 1076–1086. https://doi.org/10.1016/j.tplants.2020.05.006
Agathokleous E., Feng Z., Iavicoli I., Calabrese E.J. Nano-pesticides: a great challenge for biodiversity? The need for a broader perspective // Nano Today. 2020b. V. 30. P. 100808. https://doi.org/10.1016/j.nantod.2019.100808
Agathokleous E., Iavicoli I., Barceló D., Calabrese E.J. Ecological risks in a ‘plastic’ world: a threat to biological diversity? // J. Hazard. Mater. 2021a. V. 417. P. 126035. https://doi.org/10.1016/j.jhazmat.2021.126035
Agathokleous E., Iavicoli I., Barceló D., Calabrese E.J. Micro/nanoplastics effects on organisms: a review focusing on ‘dose’ // J. Hazard. Mater. 2021b. V. 417. P. 126084. https://doi.org/10.1016/j.jhazmat.2021.126084
Agathokleous E., Barceló D., Calabrese E.J. US EPA: opening a new window for evaluating potential sub-threshold effects and ecological risks? // Environ. Pollut. 2021c. V. 284. P. 117372. https://doi.org/10.1016/j.envpol.2021.117372
Belz R.G. Herbicide hormesis can act as a driver of resistance evolution in weeds – PSII-target site resistance in Chenopodium album L. as a case study // Pest. Manag. Sci. 2018. V. 74. P. 2874–2883. https://doi.org/10.1002/ps.5080
Belz R.G., Piepho H.P. Predicting biphasic responses in binary mixtures: pelargonic acid versus glyphosate // Chemosphere. 2017. V. 178. P. 88–98. https://doi.org/10.1016/j.chemosphere.2017.03.047
Belz R.G., Patama M., Sinkkonen A. Low doses of six toxicants change plant size distribution in dense populations of Lactuca sativa // Sci. Total Environ. 2018. V. 631–632. P. 510–523. https://doi.org/10.1016/j.scitotenv.2018.02.336
Berry R., López-Martínez G. A dose of experimental hormesis: when mild stress protects and improves animal performance // Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020. V. 242. P. 110658. https://doi.org/10.1016/j.cbpa.2020.110658
Calabrese E.J. Hormesis: why it is important to toxicology and toxicologists // Environ. Toxicol. Chem. 2008. V. 27 (7). P. 1451–1474.
Calabrese E.J. Hormetic mechanisms // Crit. Rev. Toxicol. 2013. V. 43 (7). P. 580–606. https://doi.org/10.3109/10408444.2013.808172
Calabrese E.J. Preconditioning is hormesis part I: documentation, dose–response features and mechanistic foundations // Pharmacol. Res. 2016a. V. 110. P. 242–264. https://doi.org/10.1016/j.phrs.2015.12.021
Calabrese E.J. Preconditioning is hormesis part II: how the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks // Pharmacol. Res. 2016b. V. 110. P. 265–275. https://doi.org/10.1016/j.phrs.2015.12.020
Calabrese E.J., Baldwin L.A. Chemical hormesis: its historical foundations as a biological hypothesis // Toxicol. Pathol. 1999. V. 27. P. 195–216.
Calabrese E.J., Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview // Toxicol. Appl. Pharmacol. 2005. V. 202 (3). P. 289–301. https://doi.org/10.1016/j.taap.2004.06.023
Calabrese E.J., Blain R.B. Hormesis and plant biology // Environ. Pollut. 2009. V. 157 (1). P. 42–48. https://doi.org/10.1016/j.envpol.2008.07.028
Calabrese E.J., Blain R.B. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature // Regul. Toxicol. Pharmacol. 2011. V. 61. P. 73–81. https://doi.org/10.1016/j.yrtph.2011.06.003
Calabrese E.J., Agathokleous E. Accumulator plants and hormesis // Environ. Pollut. 2021. V. 274. P. 116526. https://doi.org/10.1016/j.envpol.2021.116526
Cao Y., Yang H., Li J. et al. Sublethal effects of imidacloprid on the population development of western flower thrips Frankliniella occidentalis (Thysanoptera: Thripidae) // Insects. 2019. V. 10. P. 3. https://doi.org/10.3390/insects10010003
Chen L., Wang C., Dell B. et al. Growth and nutrient dynamics of Betula alnoides seedlings under exponential fertilization // J. Forest. Res. 2018. V. 29. P. 111–119. https://doi.org/10.1007/s11676-017-0427-2
Chouhan S., Yadav S.K., Prakash J. et al. Effect of bisphenol A on human health and its degradation by microorganisms: a review // Ann. Microbiol. 2014. V. 64. 13–21. https://doi.org/10.1007/s13213-013-0649-2
Cong M., He S., Ma H. et al. Hormetic effects of carbendazim on the virulence of Botrytis cinerea // Plant Dis. 2018. V. 102. P. 886–891. https://doi.org/10.1016/j.phrs.2015.12.020
Costa R.N., Bevilaqua N.C., Krenchinski F.H. et al. Hormetic effect of glyphosate on the morphology, physiology and metabolism of coffee plants // Plants. 2023. V. 12. P. 2249. https://doi.org/10.3390/plants12122249
Costantini D., Metcalfe N.B., Monaghan P. Ecological processes in a hormetic framework // Ecol. Lett. 2010. V. 13 (11). P. 1435–1447. https://doi.org/10.1111/j.1461-0248.2010.01531.x
Cui X., Huo M., Chen C. et al. Low concentrations of Al(III) accelerate the formation of biofilm: multiple effects of hormesis and flocculation // Sci. Total Environ. 2018. V. 634. P. 516–524. https://doi.org/10.1016/j.scitotenv.2018.03.376
De Vries W., Du E., Butterbach-Bahl K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems // Curr. Opin. Environ. Sustainab. 2014. V. 9–10. P. 90–104. https://doi.org/10.1016/j.cosust.2014.09.001
Deng D., Duan W., Wang H. et al. Assessment of the effects of lethal and sublethal exposure to dinotefuran on the wheat aphid Rhopalosiphum padi (Linnaeus) // Ecotoxicology. 2019. V. 28. P. 825–833. https://doi.org/10.1007/s10646-019-02080-8
Di Y.L., Cong M.L., Zhang R., Zhu F.-X. Hormetic effects of trifloxystrobin on aggressiveness of Sclerotinia sclerotiorum // Plant Dis. 2016. V. 100. P. 2113–2118. https://doi.org/10.1094/PDIS-03-16-0403-RE
Diaz G.J., Calabrese E., Blain R. Aflatoxicosis in chickens (Gallus gallus): an example of hormesis? // Poult. Sci. 2008. V. 87. P. 727–732.https://doi.org/10.3382/ps.2007-00403
Dormann C.F., Woodin S.J. Climate change in the arctic: using plant functional types in a meta-analysis of field experiments // Func. Ecol. 2002. V. 16. P. 4–17. https://doi.org/10.1046/j.0269-8463.2001.00596.x
Drobne D., Jemec A., Tkalec Z.P. In vivo screening to determine hazards of nanoparticles: nanosized TiO2 // Environ. Pollut. 2009. V. 157. P. 1157–1164. https://doi.org/10.1016/j.envpol.2008.10.018
Erofeeva E.A. Hormesis and paradoxical effects of wheat seedling (Triticum aestivum L.) parameters upon exposure to different pollutants in a wide range of doses // Dose—Response. 2013. V. 12 (1). P. 121–135.https://doi.org/10.2203/dose-response.13-017.Erofeeva
Erofeeva E.A. Hormesis and paradoxical effects of pea (Pisum sativum L.) parameters upon exposure to formaldehyde in a wide range of doses // Ecotoxicology. 2018. V. 27 (5). P. 569–577. https://doi.org/10.1007/s10646-018-1928-2
Erofeeva E.A. Plant hormesis and Shelford’s tolerance law curve // J. Forest. Res. 2021. V. 32. P. 1789–1802. https://doi.org/10.1007/s11676-021-01312-0
Erofeeva E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants // Sci. Total Environ. 2022. V. 804. P. 150059. https://doi.org/10.1016/j.scitotenv.2021.150059
Erofeeva E.A. Hormetic effects of abiotic environmental stressors in woody plants in the context of climate change // J. Forest. Res. 2023. V. 34. P. 7–19. https://doi.org/10.1007/s11676-022-01591-1
Fan D., Jing Y., Zhu Y. et al. Toluene induces hormetic response of soil alkaline phosphatase and the potential enzyme kinetic mechanism // Ecotoxicol. Environ. Saf. 2020. V. 206. P. 111123.
Fan D., Sun J., Liu C. et al. Measurement and modeling of hormesis in soil bacteria and fungi under single and combined treatments of Cd and Pb // Sci. Total Environ. 2021. V. 783. P. 147494. https://doi.org/10.1016/j.scitotenv.2021.147494
Foyer C.H., Rasool B., Davey J.W., Hancock R.D. Cross-tolerance to biotic and abiotic stresses in plants: a focus on resistance to aphid infestation // J. Exp. Bot. 2016. V. 67 (7). P. 2025–2037. https://doi.org/10.1093/jxb/erw079
Gopi I.K., Rattan S.I.S. Biphasic dose–response and hormetic effects of stress hormone hydrocortisone on telomerase-immortalized human bone marrow stem cells in vitro // Dose—Response. 2019. V. 17 (4). P. 1559325819889819. https://doi.org/10.1177/1559325819889819
Gu W., Liu S., Chen L. et al. Single-cell RNA sequencing reveals size-dependent effects of polystyrene microplastics on immune and secretory cell populations from zebrafish intestines // Environ. Sci. Technol. 2020. V. 54. P. 3417–3427. https://doi.org/10.1021/acs.est.9b06386
Guedes N.M.P., Tolledo J., Corrêa A.S., Guedes R.N.C. Insecticide induced hormesis in an insecticide resistant strain of the maize weevil, Sitophilus zeamais // J. Appl. Entomol. 2010. V. 134 (2). P. 142–148. https://doi.org/10.1111/j.1439-0418.2009.01462.x
Guo X., Liu M., Zhong H. et al. Responses of the growth and physiological characteristics of Myriophyllum aquaticum to coexisting tetracyclines and copper in constructed wetland microcosms // Environ. Pollut. 2020. V. 261. P. 114204. https://doi.org/10.1016/j.envpol.2020.114204
Han J., Wang S., Fan D. et al. Time-dependent hormetic response of soil alkaline phosphatase induced by Cd and the association with bacterial community composition // Microb. Ecol. 2019. V. 78. P. 961–973.
Harmsen K. A modified mitscherlich equation for rainfed crop production in semi-arid areas. 1. Theory // Wageningen J. Life Sci. 2000. V. 48 (3). P. 237–250. https://doi.org/10.1016/S1573-5214(00)80016-0
Hashmi M.Z., Naveedullah, Shen C., Yu C. Hormetic responses of food-supplied Pcb 31 to zebrafish (Danio Rerio) growth // Dose—Response. 2015. V. 13. P. 14–013. https://doi.org/10.2203/dose-response.14-013.Chaofeng
Hatfield J.L., Prueger J.H. Temperature extremes: effect on plant growth and development // Weath. Clim. Extr. 2015. V. 10. P. 4–10. https://doi.org/10.1016/j.wace.2015.08.001
Helaouët P., Beaugrand G. Physiology, ecological niches and species distribution // Ecosystems. 2009. V. 12. P. 1235–1245. https://doi.org/10.1007/s10021-009-9261-5
Jalal A., De Oliveira J.C.Jr., Ribeiro J.S. et al. Hormesis in plants: physiological and biochemical responses // Ecotoxicol. Environ. Saf. 2021. V. 207. P. 111225. https://doi.org/10.1016/j.ecoenv.2020.111225
Jia L., He X., Chen W. et al. Hormesis phenomena under Cd stress in a hyperaccumulator – Lonicera japonica Thunb // Ecotoxicology. 2013. V. 22. P. 476–485. https://doi.org/10.1007/s10646-013-1041-5
Kacienė G., Juknys R., Januškaitienė I. The role of oxidative stress in spring barley cross-adaptation to different heavy metals // Arch. Agron. Soil Sci. 2017. V. 63 (8). P. 1037–1048. https://doi.org/10.1080/03650340.2016.1256474
Kudryasheva N.S., Rozhko T.V. Effect of low-dose ionizing radiation on luminous marine bacteria: radiation hormesis and toxicity // J. Environ. Radioact. 2015. V. 142. P. 68–77. https://doi.org/10.1016/j.jenvrad.2015.01.012
Li B., Ding Y., Cheng X. et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice // Chemosphere. 2020. V. 244. P. 125492. https://doi.org/10.1016/j.chemosphere.2019.125492
Lo H.S., Po B.H.K., Li L. et al. Bisphenol A and its analogues in sedimentary microplastics of Hong Kong // Mar. Pollut. Bull. 2021. V. 164. P. 112090. https://doi.org/10.1016/j.marpolbul.2021.112090
Martínez J.L. Effect of antibiotics on bacterial populations: a multi-hierachical selection process // F1000Res. 2017. V. 6. P. 51. https://doi.org/10.12688/f1000research.9685.1
Martinez-Medina A., Flors V., Heil M. et al. Recognizing plant defense priming // Trends Plant Sci. 2016. V. 21 (10). P. 818–822. https://doi.org/10.1016/j.tplants.2016.07.009
McClure C.D., Zhong W., Hunt V.L. et al. Hormesis results in trade-offs with immunity // Evolution. 2014. V. 68. P. 2225–2233. https://doi.org/10.1111/evo.12453
Mitton F.M., Miglioranza K., Gonzalez M. et al. Assessment of tolerance and efficiency of crop species in the phytoremediation of DDT polluted soils // Ecol. Eng. 2014. V. 71. P. 501–508. https://doi.org/10.1016/j.ecoleng.2014.07.069
Moore M.N., Shaw J.P., Adams D.R.F., Viarengo A. Anti-oxidative cellular protection effect of fasting-induced autophagy as a mechanism of hormesis // Mar. Environ. Res. 2015. V. 107. P. 35–44. https://doi.org/10.1016/j.marenvres.2015.04.001
Morkunas I., Woźniak A., Mai V.C. et al. The role of heavy metals in plant response to biotic stress // Molecules. 2018. V. 23 (9). P. 2320. https://doi.org/10.3390/molecules23092320
Morse J.G. Agricultural implications of pesticide-induced hormesis of insects and mites // Hum. Exp. Toxicol. 1998. V. 17 (5). P. 266–269. https://doi.org/10.1177/096032719801700510
Mortimer M., Kasemets K., Kahru A. Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila // Toxicology. 2010. V. 269. P. 182–189. https://doi.org/10.1016/j.tox.2009.07.007
Motai A., Terada Y., Kobayashi A. et al. Combined effects of irrigation amount and nitrogen load on growth and needle biochemical traits of Cryptomeria japonica seedlings // Trees. 2017. V. 31. P. 1317–1333. https://doi.org/10.1007/s00468-017-1551-5
Nielsen M.E., Roslev P. Behavioral responses and starvation survival of Daphnia magna exposed to fluoxetine and propranolol // Chemosphere. 2018. V. 211. P. 978–985. https://doi.org/10.1016/j.chemosphere.2018.08.027
Obodovskiy I. Radiation: fundamentals, applications, risks, and safety. Amsterdam: Elsevier, 2019. 720 p.
Pincelli-Souza R.P., Bortolheiro F.P., Carbonari C.A. et al. Hormetic effect of glyphosate persists during the entire growth period and increases sugarcane yield // Pest Manag. Sci. 2020. V. 76. P. 2388–2394. https://doi.org/10.1002/ps.5775
Rahavi M.R., Migicovsky Z., Titov V., Kovalchuk I. Transgenerational adaptation to heavy metal salts in Arabidopsis // Front. Plant Sci. 2011. V. 2. P. 91. https://doi.org/10.3389/fpls.2011.00091
Rustad L.E., Campbell J.L., Marion G.M. et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming // Oecologia. 2001. V. 126. P. 543–562. https://doi.org/10.1007/s004420000544
Roberts A.P., Mount A.S., Seda B. et al. In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna // Environ. Sci. Technol. 2007. V. 41. P. 3025–3029. https://doi.org/10.1021/es062572a
Schirrmacher V. Less can be more: the hormesis theory of stress adaptation in the global biosphere and its implications // Biomedicines. 2021. V. 9 (3). P. 293. https://doi.org/10.3390/biomedicines9030293
Schreck C.B. Stress and fish reproduction: the roles of allostasis and hormesis // Gen. Comp. Endocrinol. 2010. V. 165. P. 549–556. https://doi.org/10.1016/j.ygcen.2009.07.004
Selye H. Stress without distress. N.Y.: Harper, Row, 1974. 171 p.
Shahid M., Niazi N.K., Rinklebe J. et al. Trace elements-induced phytohormesis: a critical review and mechanistic interpretation // Crit. Rev. Environ. Sci. Technol. 2020. V. 50 (19). P. 1984–2015. https://doi.org/10.1080/10643389.2019.1689061
Shelford V.E. Animal communities in a temperate America. Chicago: Univ. Chicago Press, 1913. 386 p.
Sial M.U., Zhao Z., Zhang L. et al. Evaluation of insecticides induced hormesis on the demographic parameters of Myzus persicae and expression changes of metabolic resistance detoxification genes // Sci. Rep. 2018. V. 8 (1). P. 16601.
Simkin S.M., Allen E.B., Bowman W.D. et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States // PNAS USA. 2016. V. 113. P. 4086–4091. https://doi.org/10.1073/pnas.1515241113
Smith S.D., Huxman T.E., Zitzer S.F. et al. Elevated CO2 increases productivity and invasive species success in an arid ecosystem // Nature. 2000. V. 408. P. 79–82. https://doi.org/10.1038/35040544
Tang S., Liang J., Xiang C. et al. A general model of hormesis in biological systems and its application to pest management // J. R. Soc. Interface. 2019. V. 16. P. 20190468.
Vaiserman A., Cuttler J.M., Socol Y. Low-dose ionizing radiation as a hormetin: experimental observations and therapeutic perspective for age-related disorders // Biogerontology. 2021. V. 22. P. 145–164. https://doi.org/10.1007/s10522-020-09908-5
Walter J., Jentsch A., Beierkuhnlein C., Kreyling J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes // Environ. Exp. Bot. 2013. V. 94. P. 3–8. https://doi.org/10.1016/j.envexpbot.2012.02.009
Wang S., Huang B., Fan D. et al. Hormetic responses of soil microbiota to exogenous Cd: a step toward linking community-level hormesis to ecological risk assessment // J. Hazard. Mater. 2021. V. 416. P. 125760. https://doi.org/10.1016/j.jhazmat.2021.125760
Wiegant F.A., Prins H.A., van Wijk R. Postconditioning hormesis put in perspective: an overview of experimental and clinical studies // Dose—Response. 2011. V. 9 (2). P. 209–224.
Wu Z., Dijkstra P., Koch G.W. et al. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation // Glob. Biol. 2011. V. 17. P. 927–942. https://doi.org/10.1111/j.1365-2486.2010.02302.x
Xu Y., Liu S., Chen F., Wang Z. pH affects the hormesis profiles of personal care product components on luminescence of the bacteria Vibrio qinghaiensis sp.-Q67 // Sci. Total Environ. 2020. V. 713. P. 136656.
Xu Z., Hu T., Zhang Y. Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China // Eur. J. Forest Res. 2012. V. 131. P. 811–819. https://doi.org/10.1007/s10342-011-0554-9
Yi M., Zhou S., Zhang L., Ding S. The effects of three different microplastics on enzyme activities and microbial communities in soil // Water Environ. Res. 2021. V. 93. P. 24–32. https://doi.org/10.1002/wer.1327
Zhang C., Li C., Chen S. et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways // Sci. Rep. 2017. V. 7. P. 41082. https://doi.org/10.1038/srep41082
Zhang R., Zhang Y., Xu Q. et al. Hormetic effects of mixtures of dimethachlone and prochloraz on Sclerotinia sclerotiorum // Plant Dis. 2019. V. 103 (3). P. 546–554. https://doi.org/10.1094/PDIS-06-18-1071-RE
Zied D.C., Dourado F.A., Dias E.S., Pardo-Giménez A. First study of hormesis effect on mushroom cultivation // World J. Microbiol. Biotechnol. 2017. V. 33 (11). P. 195. https://doi.org/10.1007/s11274-017-2342-2
Дополнительные материалы отсутствуют.
Инструменты
Успехи современной биологии



