Ботанический журнал, 2021, T. 106, № 9, стр. 898-901
EMBRYOLOGY OF DYPSIS DECARYI (ARECACEAE)
H. N. Krishna Kumar *
Department of Studies in Botany, Pooja Bhagavat Memorial Mahajana Education Centre, PG wing of SBRR Mahajana First Grade College (Autonomous), Affiliated to University of Mysore
K.R.S. Road, Metagalli Mysore-570 016, Karnataka, India
* E-mail: hnkrishnakumars@gmail.com
Поступила в редакцию 11.03.2020
После доработки 7.05.2021
Принята к публикации 1.06.2021
Аннотация
The paper deals with the study of microsporogenesis and male gametophyte development, megasporogenesis and female gametophyte development in Dypsis decaryi (Jum.) Beentje et J. Dransf. The anther is tetrasporangiate and each sporangium is encircled by wall layers of an outer epidermis and an inner glandular tapetum. Immediately below the epidermis, an endothecium is present. Two middle layers are located between endothecium and tapetum. Simultaneous quadripartition in the pollen mother cells results in tetrads. The microspore tetrads are isobilateral and tetrahedral. Rarely polyspory has been noticed. The pollen grains are 2-celled when shed. The ovary is superior, tricarpellary and syncarpous. The ovules are bitegmic and crassinucellate. The sporogenous cell directly develops into megaspore mother cell. Seldom two megasporocytes are met with in a single ovule. The megaspore tetrad is linear. The development of the female gametophyte follows the Polygonum type.
The Arecaceae is a large family with about 212 genera and 3000 species (Takhtajan, 1987). In India, there are about 27 genera and 91 species (Ahmedullah, Nayar, 1986). The members of the Arecaceae commonly called palms are chiefly distributed in the tropical and subtropical belts of the world. They thrive in diverse habitats such as rainforests, deserts and semi-arid regions. Majority of the Arecaceae species are considered economically very important. Palms are also finding their rightful place among the ornamental plants of the world. India has large estates of coconut palms, betel nut palms and recently oil palms. Embryo-logical studies on Arecaceae members are very few, incomplete and sometimes doubtful (Maheshwari, 1955; Haccius, Philip, 1979). Only a few workers have been able to work out some fragmentary accounts of the reproductive biology of a few species of palms (Davis, 1966; Johri et al., 1992).
The genus Dypsis belonging to the family Arecaceae comprises about 14 species. Dypsis decaryi is commonly known as Triangle Palm. This is a very striking palm, with the leaves arising from three distinct points around the trunk, hence the common name. This solitary-trunked palm can grow up to 25 feet. The tall, stiff feather-shaped leaves are up to 12 feet long. The Triangle Palm is native to the island of Madagascar, which is off the southeast coast of Africa. The habitat of this palm is wide spread from open fields to tropical rainforests at both high and low elevations. This is an important ornamental palm grown in gardens.
The embryology of thousands of palm species remains uninvestigated. Therefore, to fill the gap in our knowledge on the embryology of palms, the present work has been undertaken.
MATERIALS AND METHODS
Materials for the present investigation included staminate and pistillate flowers of Dypsis decaryi collected from Lal Bagh Botanical Garden, Bangalore, Karnataka. The floral materials were fixed in formalin-acetic acid-alcohol. The flowers at different stages of development were dehydrated in a graded ethanol-xylol series. Paraffin infiltration and embedding were done. The sections were cut at 8–14 μm thickness using Spencer rotary microtome. The paraffin ribbons containing sections were affixed onto the slides using egg albumen as adhesive. The micropreparations were processed following the customary method of alcohol-xylene series and stained with Heidenhein’s iron alum and haemetoxylin, counterstained with erythrosine in clove oil and mounted using DPX mountant.
RESULTS
Microsporogenesis and the development of male gametophyte
Each staminate flower has six stamens. A cross section of the male flower shows the section of six anthers with pistillode in the centre (Fig. 1a). Around the pistillode and connective region of the anther, tannin-filled cells have been observed. The anther is quadrilocular and each locule is encircled by wall layers (Fig. 1b). The wall layers consist of an outer epidermis and an inner glandular tapetum, the latter surrounding developing pollen mother cells and nourishing them. Immediately below the epidermis an endothecium is present. The cells of the endothecium are vacuolated, and it develops fibrous bands when the anther attains maturity. Generally, the cells are uninucleate; however, some of the cells contain two nuclei. In between endothecium and tapetum there are two middle layers, which are crushed and absorbed by pollen mother cells during their development (Fig. 1c).
Fig. 1.
Dypsis decaryi
(a) transverse section (TS) of a male flower to show 6 anthers and a pistillode, ×50.
(b) young microsporangium to show sporogenous tissue; TS, ×400.
(c) microsporangium to show pollen mother cells; TS, ×400.
(d) part of microsporangium to show meiosis-I in pollen mother cells (arrows); TS, ×400.
(e, f) part of microsporangium to show tetrads. Polyspory (arrow); TS, ×400.
(g) part of microsporangium at microspore stage; TS, ×400.
(h) part of microsporangium to show pollen at the time of shedding; TS, ×400.
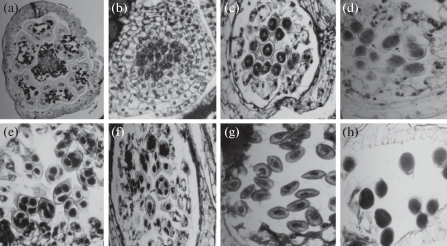
Simultaneous quadripartition in the pollen mother cells results in tetrads and the cytokinesis takes place by cell plate formation (Fig. 1d, e). The microspore tetrads are isobilateral and tetrahedral (Fig. 1e). Rarely polyspory was noticed (Fig. 1f). The microspores separate from the tetrads and grow in size with dense cytoplasm and nucleus at the centre (Fig. 1g). The microspore nucleus divides to form a rounded vegetative cell and a lenticular generative cell. In the beginning each microsporangium organizes its own stomium but at later stages a general stomium of thick-walled cells is formed between adjacent microsporangia. The anther dehisces at the region of stomium and releases pollen grains. The pollen grains are 2-celled when shed (Fig. 1h).
Megasporogenesis and female gametophyte development
The ovary is superior, tricarpellary and syncarpous. The stylar canal as well as the locular extensions are lined by radially elongated glandular cells. The primordium of the ovule is of basal origin (Fig. 2a). The ovules are bitegmic and crassinucellate. They are hemianatropous and transverse. The micropyle is straight and formed by both the integuments. The embryo sac expands uniformly all over and is ovoid with broad micropylar and antipodal ends. The cells around the antipodal end of the embryo sac become thick-walled and prevent its further expansion while the micropylar part expands considerably.
Fig. 2.
Dypsis decaryi
(a) longitudinal section (LS) of part of a female flower to show the position of ovule at the megaspore mother cell stage, ×400.
(b) part of ovule to show megaspore mother cell; LS, ×400.
(c) part of ovule to show double megaspore mother cells (arrow); LS, × 400.
(d) part of ovule to show functional megaspore at prophase stage; LS, ×400.
(e) part of ovule to show 2-nucleate embryo sac; LS, ×400.
(f) part of ovule to show 4-nucleate embryo sac; LS, ×400.
(g) part of ovule to show egg and antipodals; LS, ×400.
(h) part of ovule to show secondary nucleus (arrow); LS, ×400.
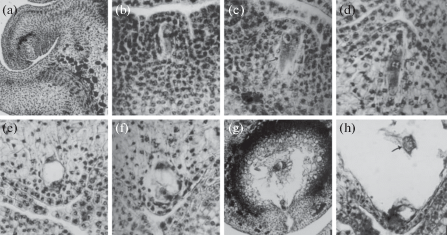
One of the hypodermal cells in the ovular primordium differentiates as the archesporium. It cuts off an outer 2–3 layers of parietal cells and an inner sporogenous cell. Later the sporogenous cell directly develops into megaspore mother cell (Fig. 2b). Occassionally two megasporocytes are found in a single ovule (Fig. 2c). The megaspore tetrad is linear. The three megaspores of the micropylar side degenerate and the chalazal megaspore is functional (Fig. 2d). The nucleus of the functional megaspore undergoes three successive mitotic divisions leading to the formation of 8-nucleate embryo sac (Fig. 2e, f). Thus the development of the female gametophyte follows the Polygonum type (Maheshwari, 1950). The mature embryo sac contains an egg apparatus, central cell with secondary nucleus and antipodals (Fig. 2g, h). The synergids show hooks on their free sides. The antipodals are persistent and become aggressive. They are small and uninucleate at first but become large and multinucleate in the mature embryo sac (Fig. 2g).
DISCUSSION
The pattern of development of the microsporangium wall follows the monocotyledonous type (Davis, 1966). The tapetum and the middle layers are the sisters. The tapetum is of the secretory type as in the other investigated species of palms (Johri et al., 1992; Robertson, 1976a; Krishna Kumar, Ramaswamy, 2003.). In the present work, five-layered anther walls have been noticed with two middle layers. However, Juliano and Quisumbing (1931) have observed a 6–8-layered anther wall in Cocos nucifera. In Hyphaene indica, Mahabale and Chennaveeraiah (1957) have found a 5–6-layered anther wall.
In the present study it has been found that the tapetal cells are uninucleate initially. Subsequently, they become binucleate. The cells start disorganizing only when dyads and tetrads are formed. This has also been observed in earlier works on palms (Rao, 1959a, b; Mahabale, Biradar, 1968).
The endothecium develops fibrous secondary wall thickenings as in the other investigated species of Palms (Johri et al., 1992). The cells of the connective completely filled with tannins in the early stages of development itself as in the tribe Ceroxylinae (Rao, 1959b). In the young microsporangium, the epidermal layer is very prominent. During the development, it becomes enucleate and only its remnants are observed in the mature microsporangium. The division of the pollen mother cells is of the simultaneous type as reported by Rao (1959b).
The microspore tetrads are isobilateral and tetrahedral as in Phoenix (Biradar, 1968; Biradar, Mahabale, 1968) and Caryota urens (Shirke, Mahabale, 1972). A rare occasion of polyspory has been observed like in Hyphaene indica (Mahabale, Chennaveeraiah, 1957). Generally, a stomium of thick-walled cells is organized between the adjacent microsporangia. The anther dehisces at the region of stomium and releases pollen grains as in other investigated palms (Johri et al., 1992). However, in the present study each microsporangium organizes its own stomium at the first stage and a general stomium between two adjacent microsporangia is organized at the end. Mature pollen grains are shed 2-celled as in most investigated palm species (Johri et al., 1992). However, in Calamus gamblei and C. rotang the pollen grains are 2–3-celled at the time of shedding (Krishna Kumar, Ramaswamy, 2003).
The ovary is superior, tricarpellary, syncarpus, trilocular and with a single ovule in each locule on axile placenta as in other investigated species of palms (Johri et al., 1992; Robertson, 1976b). At the early stages, all the 3 ovules appear to be functional, but at the later stages of development, only one ovule is functional and gives rise to embryo sac. The ovules are bitegmic, crassinucellate and hemianatropous. The same has been reported by Rao (1959b) in Chrysalidocarpus and Areca. In Hyphaene indica Mahabale and Chennaveeraiah (1957) observed orthotropous ovules. The integumentary tapetum like cells have been observed in species of Cocos and Areca concinna (Rao, 1959a, b), Phoenix sylvestris (Mahabale, Biradar, 1968) and Livistona chinensis (Kulkarni, Mahabale, 1974).
A single hypodermal cell differentiates as the archesporium in the ovular primordium. The archesporial cell divides periclinally to produce a parietal cell and a sporogenous cell which directly functions as megaspore mother cell as in majority of the investigated Arecaceae (Davis, 1966; Johri et al., 1992). However, Quisumbing and Juliano (1927) reported tenuinucellate ovules in Cocos nucifera. There is a single megaspore mother cell in each ovule. Occasionally two megaspore mother cells have been noticed in each ovule. The same has been reported in Elaeis guineensis (De Poerck, 1950) and Phoenix sylvestris (Mahabale, Biradar, 1968). Kajale and Ranade (1953) have observed twin embryo sacs in Elaeis guineensis. The megaspore tetrads are linear as in most investigated palm species (Johri et al., 1992). Rao (1959a) observed both linear and T-shaped tetrads in Caryota mitis, Chrysalidocarpus lutescens, and Shirke and Mahabale (1972) in Caryota urens. Kajale and Ranade (1953) noted four different kinds of megaspore tetrads in Elaeis guineensis. The mode of development of female gametophyte conforms to the monosporic 8-nucleate Polygonum type of Maheshwari (1950) as in majority of the investigated palms (Johri et al., 1992). However, bisporic 8-nucleate Allium type of female gametophyte development has been reported in Hyphaene indica (Mahabale, Chennaveeraiah, 1957). The antipodals are sitting on nucellar tissue that projects into the embryosac. They are aggressive and polyploidization has been noticed. The same has been observed by (Rao 1959b) in Chrysalidocarpus, Howea and Actinophloeus.
Список литературы
Ahmedullah M., Nayar M.P. 1986. Endemic plants of the Indian region Vol. 1. Botanical survey of India. Calcutta. P. 203–204.
Biradar N.V. 1968. Studies on Palms: Embryology of Phoenix pusila Gaertn., P. acaulis Buch. and P. reclinata Jacq. – Proc. Ind. Acad. Sci. 67: 165–173.
Biradar N.V., Mahabale T.S. 1968. Studies on Palms: Embryology of Phoenix robusta Hook. – Proc. Ind. Acad. Sci. 67B: 1–8.
Davis G.L. 1966. Systematic embryology of Angiosperms. New York. 528 p.
De Poerck R.A. 1950. Contribution a petude de Palmier a huile Africain Elaeis guineensis. – Oleagincux. 5: 623–628.
Haccius B., Philip V.J. 1979. Embryo development in Coccos nucifera L. A critical contribution to a general understanding of Palm embryogenesis. – Plant Syst. Evol. 132: 91–106.
Johri B.M., Ambegaokar K.B., Srivastava P.S. 1992. Comparative Embryology of Angiosperms. 2. Berlin. P. 944–950.
Juliano J.B., Quisumbing E. 1931. Morphology of the male flower of Cocos nucifera L. – Philipp. J. Sci. 45: 449–458.
Kajale L.B., Ranade S.G. 1953. The embryo sac of Elaeis guineensis Jacq. A reinvestigation. – J. Indian Bot. Soc. 32: 101–107.
Krishna Kumar H.N., Ramaswamy S.N. 2003. Contributions to the Study of Microsporogenesis in Calamus L. (Arecaceae). – Taiwania. 48 (3): 180–193.
Kulkarni K.M., Mahabale T.S. 1974. Studies on Palms: Embryology of Livistona chinensis R. Br. – Proc. Ind. Acad. Sci. 67: 1–17.
Mahabale T.S., Chennaveeraiah M.S. 1957. Studies on Hyphaene indica Becc. I. Morphology. – Phytomorphology. 7: 184–194.
Mahabale T.S., Biradar N.V. 1968. Studies on palms: Embryology of Phoenix sylvestris Roxb. – Proc. Ind. Acad. Sci. 67 (2) B: 77–96.
Maheshwari P. 1950. An introduction to the Embryology of Angiosperms. New York, London. 453 p.
Maheshwari P. 1955. The occurrence of bisporic embryo sacs in Angiosperms – A critical review. – Phytomorphology. 5: 67–69.
Quisumbing E., Juliano J.B. 1927. Development of Ovule and Embryo sac of Cocos nucifera. – Bot. Gaz. 84: 279–293.
Rao C.V. 1959a. Contributions to the embryology of Palmae I. Sabaleae. – Proc. Nat. Inst. Sci. India. 29: 134–164.
Rao C.V. 1959b. Contributions to the embryology of Palmae II. Ceroxylineae. – J. Indian. Bot. Soc. 37: 47–75.
Robertson B.L. 1976a. Embryology of Jubaeopsis caffra Becc. 1. Microsporangium, Microsporogenesis and Microgametogenesis. – J. S. Afr. Bot. 42: 97–108.
Robertson B.L. 1976b. Embryology of Jubaeopsis caffra Becc. 2. Megasporangium, Megasporogenesis and Megagametogenesis. – J. S. Afr. Bot. 42: 173–184.
Shirke N.S., Mahabale T.S. 1972. Studies on palms: Embryology of Caryota urens L. – In: Murthy Y.S., Johri B.M., Mohan Ram H.Y., Varghese T.M. (eds). Advances in Plant Morphology. Sarita Prakashan, Meerut. P. 218–232.
Takhtajan A. 1987. Systema Magnoliophytorum. Moscow. 439 p. (In Russ.).
Дополнительные материалы отсутствуют.
Инструменты
Ботанический журнал


