Микробиология, 2022, T. 91, № 6, стр. 666-684
На краю радуги: длинноволновые хлорофиллы и фотосинтетическая адаптация цианобактерий к дальнему красному свету
А. В. Пиневич a, *, С. Г. Аверина a
a Санкт-Петербургский государственный университет
199034 Санкт-Петербург, Россия
* E-mail: Pinevich.A@mail.ru
Поступила в редакцию 25.05.2022
После доработки 11.07.2022
Принята к публикации 12.07.2022
- EDN: ORWIRG
- DOI: 10.31857/S0026365622600444
Аннотация
Феномен фотосинтетической адаптации цианобактерий к дальнему красному свету (ДКС; 700‒750 нм) тесно связан с такими фундаментальными вопросами, как фототрофия, микробная экология и разнообразие бактерий. В практическом плане данная биоэнергетическая стратегия важна для биотехнологии фотосинтеза, с перспективой получить доступ к дополнительному ресурсу световой энергии. Как известно, большинство цианобактерий использует свет длиной волны 400‒700 нм; возбужденное состояние поступает от светособирающего комплекса к хлорофиллу a (Хл a) реакционных центров двух фотосистем с красными максимумами поглощения ∼700 нм. После выделения первых штаммов с хлорофиллами d и f выяснилось, что цианобактерии могут использовать и ДКС. В настоящее время получен значительный объем данных о цианобактериях, конститутивно образующих Хл d, а также о тех, которые синтезируют Хл f или Хл f/Хл d при фотоакклиматизации к ДКС (англ. far-red light photoacclimation; FaRLiP). Включение этих пигментов в состав фотосинтетического аппарата, в частности с использованием механизма FaRLiP, повышает адаптационный потенциал и расширяет границы распространения цианобактерий. В обзоре приводятся сведения о фотосинтетическом аппарате с Хл d или Хл d/Хл f, о генном кластере FaRLiP, о разнообразии и филогении цианобактерий с конститутивным или индуцированным синтезом длинноволновых хлорофиллов, об использовании хлорофиллов в качестве хемотаксономического признака и отражении этого признака в номенклатуре цианобактерий.
Свет ‒ разнообразный источник энергии для фототрофии, по аналогии с разнородными субстратами, используемыми при хемотрофии. В этом отношении квантоспецифичные пигмент-белковые комплексы и хромопротеины аналогичны субстратспецифичным ферментам. Неслучайно термины “апопротеин” и “апофермент” сходны не только по звучанию, но и по смыслу (греч. apo- в данном случае: быть в основе; соответственно, белковый компонент фоторецептора и ферментный белок).
Подобно разным химическим субстратам, кванты в разных участках световой шкалы неравноценны в физиологическом отношении. Ультрафиолетовые кванты (λ < 400 нм) относительно богаты энергией, однако она не только не ассимилируется, но и вызывает разрушение (выцветание) фотосинтетических пигментов. Инфракрасные кванты (λ > 750), напротив, относительно бедны энергией; тем не менее, некоторые аноксигенные бактерии способны ее ассимилировать. Использовать всю радугу от фиолетового (λ ∼ 400 нм) до красного (λ ∼ 700 нм) края могут цианобактерии так называемой Сrown группы и архаичного класса Gloeobacteria (Pinevich, Averina, 2021). Некоторым цианобактериям доступен и дальний красный свет (ДКС; 700‒750 нм). Как следствие, цианобактерии могут заселять световые ниши в интервале 400‒750 нм, используя механизмы фотосинтетической адаптации.
Фотосинтетической адаптацией (англ. photoadaptation) называется приспособляемость к режимам светового излучения разной интенсивности (количественные стратегии) или разного спектрального состава (качественные стратегии). Первые стратегии объединяет установление обратной зависимости между эффективной площадью светособирающей антенны и потоком светового излучения: физиологический оптимум достигается за счет изменения протяженности фотосинтетических мембран, а также плотности упаковки фотосинтетических единиц и их размера (Drews, Niederman, 2002). К качественным стратегиям относятся: переход Состояние 1 ↔ Состояние 2 (Allen, 1992), комплементарная хроматическая адаптация (Grossman et al., 1993) и фотосинтетическая адаптация к ДКС (Averina et al., 2019).
Хотя кванты длиной волны >700 нм относительно бедны энергией, некоторые бактерии способны образовывать “длинноволновые” (англ. red-shifted) металлопорфирины ‒ хлорофиллы (Хл) и бактериохлорофиллы ‒ и так модифицировать фотосинтетический аппарат, что адаптация к нишам, где много ДКС, становится выгодной метаболической стратегией (Kühl et al., 2005).
Инфракрасный свет используют пурпурные бактерии, образующие бактериохлорофиллы a или b, длинноволновые максимумы которых находятся, соответственно, при 800‒900 и 980‒1050 нм (Deisenhofer et al., 1985). Максимумы ≤800 нм имеют бактериохлорофилл g у гелиобактерий (Neerken, Amesz, 2001) и бактериохлорофиллы с, d, e и f у зеленых бактерий и хлорацидобактерий (Amesz, Neerken, 2002; Bryant et al., 2007).
Светособирающий комплекс (ССК) у большинства цианобактерий поглощает видимый свет, и возбужденное состояние мигрирует к Хл a в реакционных центрах (РЦ) двух фотосистем (ФС) ‒ ФС I и ФС II с максимумами поглощения, соответственно, при 700 и 680 нм. Возможность фотосинтетической адаптации к ДКС у цианобактерий не предполагалась до тех пор, пока у них не были обнаружены длинноволновые Хл d и Хл f (Miyashita et al., 1996; Chen et al., 2010). Данная биоэнергетическая стратегия быстро стала темой многих исследований, результаты которых обобщены в обзорах литературы, публиковавшихся почти ежегодно с начала 2010-х годов (Loughlin et al., 2013; Gan, Bryant, 2015; Gan et al., 2015; Li, Chen, 2015; Allakhverdiev et al., 2016; Badshah et al., 2017; Averina et al., 2018, 2019; Sawicki, Chen, 2020; Friedrich, Schmitt, 2021). Настоящее сообщение продолжает эту серию, по-новому обобщая и реструктурируя материал, с учетом последних данных.
ДЛИННОВОЛНОВЫЕ ХЛОРОФИЛЛЫ
Хлорофиллы, или магниевые хелаты двойного эфира хлорофиллиновой кислоты с фитолом и метанолом, интенсивно поглощают синий свет (т.н. полоса Soret) и красный свет (Q-полоса, или Qy-вектор энергетического перехода); длинноволновые хлорофиллы интенсивно поглощают синий свет и ДКС (рис. 1). Положение максимумов на спектрах поглощения in situ или у изолированных пигмент-белковых комплексов иное, чем у препаратов соответствующих хлорофиллов (French, 1960): в частности, красный максимум перемещается к большей длине волны. В очередности их описания хлорофиллы обозначают буквами от a до f (кроме буквы e, которая была вынужденно пропущена после доказательства ошибочной идентификации “Хл e” у красных водорослей; см.: Larkum et al., 2018).
Рис. 1.
Структурные формулы длинноволновых хлорофиллов (верхний ряд) и спектры поглощения в 100%-ном ацетоне (нижний ряд; см. Averina et al., 2019).
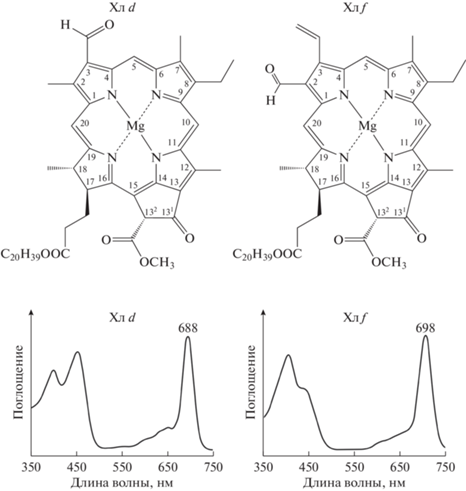
В функциональном отношении хлорофиллы подразделяют на главные и вспомогательные: первые входят в реакционные центры, вторые в ‒ ССК. Посредником между вспомогательными и главными хлорофиллами служат малые кор-антенны реакционных центров. Как автономно действующие, они неэффектвны: даже при максимальном солнечном освещении (на экваториальном высокогорье в летний полдень) частота их перехода в возбужденное состояние уступила бы скорости транспорта электронов в реакционных центрах, что затормозило бы фотосинтез (Hunter et al., 1989). Это не происходит благодаря присутствию ССК ‒ фотосинтетической антенны с намного большей эффективной площадью (Blankenship, Chen, 2013).
Как правило, цианобактерии образуют только один хлорофилл ‒ Хл a, который входит в состав кор-антенн и реакционных центров; функцию ССК выполняет высокоупорядоченный агрегат фикобилипротеинов ‒ фикобилисома (ФБС) (Grossman et al., 1993). Небольшая группа цианобактерий-прохлорофитов (Пиневич и соавт., 2010) использует вместо ФБС хлорофилл-белковый комплекс, содержащий либо Хл a/Хл b, либо Хл a2/Хл b2 (3,8-дивинильные производные Хл a и Хл b). В число порфиринов ССК цианобактерий также может входить минорный Хл с-подобный пигмент (Mg-3,8-дивинил протохлорофиллид, Mg-ДВП), обнаруженный у некоторых прохлорофитов и Хл d-содержащей цианобактерии Acaryochloris marina (Averina et al., 2019). Что касается длинноволновых Хл d и Хл f, то первый из них входит в состав не только в ССК, но и реакционных центров, а второй – в ССК и, возможно, реакционные центры.
У земной поверхности поток солнечной радиации примерно одинаков в диапазонах 600–700 и 700–800 нм. Но, поскольку длинноволновые кванты беднее энергией, чем коротковолновые, фотосинтез при >700 нм менее выгоден, чем в видимой части спектра, и цианобактерии обычно не используют ДКС. Тем не менее, некоторые из них образуют длинноволновые Хл d и Хл f, и зона фотосинтетически активной радиации (ФАР) распространяется на длинноволновую часть спектра (Kühl et al., 2005). Однако в водоемах глубина проникновения света обратно пропорциональна длине волны; инфракрасный свет полностью поглощается в нескольких сантиметрах от поверхности. Соотношение красного света и ДКС в морской или чистой озерной воде линейно возрастает с глубиной (Kirk, 1994), причем ДКС проникает не дальше 10 м (Gan et al., 2014). Поэтому адаптированные к ДКС цианобактерии, как правило, не заселяют верхние этажи эуфотической зоны морей и континентальных водоемов, а образуют биопленки на (суб)литорали, на почве и на каменистых субстратах.
Хлорофилл d
Структура и оптические свойства. В молекуле Хл d положение С3 занимает формильная, а не винильная группа, как в молекуле Хл a (рис. 1). Соответственно, Q-полоса смещена в длинноволновую область (665 → 688 нм, в 100%-ном ацетоне; см. Averina et al., 2019).
Открытие Хл d часто связывают с анализом пигментов Rhodophyceae (Manning, Strain, 1943). Позднее оказалось (Holt, 1961), что опубликованный спектр поглощения совпадает со спектром поглощения 3-девинил-3-формил Хл a, по-видимому, образовавшегося из-за погрешности методики фракцонировании образца. С другой стороны, “загадку” открытия Хл d объясняют присутствием данного пигмента у бактериальных симбионтов Rhodophyceae (Wood, 2012; Kiang et al., 2022). Так или иначе, впервые аутентичный Хл d был обнаружен у A. marina (Miyashita et al., 1996).
Хл d и не содержащие магния производные ‒ феофитин d и пирофеофитин d, находят в морских илах Японских островов, на пелагиали Берингова моря, в соленых озерах Антарктиды и некоторых пресных озерах, в частности, в крупнейшем озере Японии Бива (Kashiyama et al., 2008). Если специфические прозводные Хл d можно получить в лаборатории, то в природе такая химическая реакция маловероятна, особенно для пирофеофитина; скорее всего, эти порфирины являются побочными продуктами микробного катаболизма Хл d и/или Хл а. В противном случае эти данные можно интерпретировать как косвенное свидетельство широкого распространения Хл d.
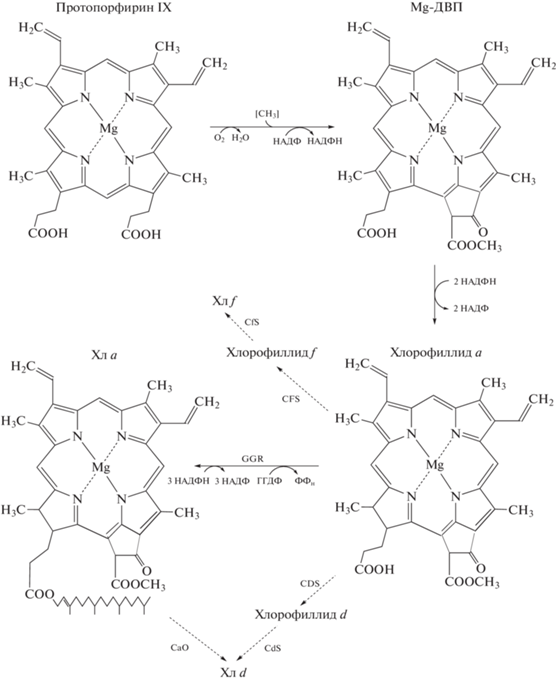
Биосинтез. Индивидуальные представители молекулярного семейства (бактерио)хлорофиллов образуются на завершающих этапах глобального пути биосинтеза металлопорфиринов (Bauer et al., 1993). Для Хл d теоретически возможны два способа: 1) посредством прямого превращения Хл a → Хл d, поскольку эти молекулы различаются только одним боковым заместителем; 2) на ответвлении магистрального пути (Loughlin et al., 2013). Первый механизм более реален (рис. 2): в опытах с культурами Acaryochloris marina, выращенными на среде с добавлением 18О2 или ${\text{H}}_{2}^{{\,\,\,18}}{\text{O,}}$ установлено, что кислородный атом формильной группы происходит из молекулярного кислорода, а не из воды (Schliep et al., 2010). Поскольку синтаза Хл d не обнаружена, есть предположение, что оксидазную реакцию неспецифически катализирует либо цитохром P450-типа (обычный участник монооксигеназных реакций), либо специальная оксигеназа для феофорбида a (Chen, Blankenship, 2011; Yoneda et al., 2016). В опытах in vitro показано, что в образовании Хл d участвуют ферменты со свободной тиольной группой, например, цистеиновая протеаза папаин (Koizumi et al., 2005) или низкомолекулярные тиосоединения (Fukusumi et al., 2012). То, что данное превращение связано с тиолсодержащими белками или с малыми молекулами с HS-группой, подтверждается транскриптомным анализом штамма дикого типа Chlorogloeopsis fritschii PCC 9212 и мутантов по генам регуляторных белков RfpA, RfpB и RfpC (Ho, Bryant, 2019). В частности, в образовании затравки Хл d для сборки ФС II могут участвовать богатые остатками Cys субъединицы фикобилипротеинов (Bryant et al., 2020).
Рис. 2.
Схема конечных этапов биосинтеза длинноволновых хлорофиллов. Предполагаемые реакции выделены пунктиром. Обозначения: Mg-ДВП ‒ Mg-3,8-дивинил протохлорофиллид; CaO ‒ хлорофилл a оксигеназа; CDS ‒ хлорофиллид d синтаза; CdS ‒ хлорофилл d синтаза; CFS ‒ хлорофиллид f синтаза; CfS ‒ хлорофилл f синтаза; GGR ‒ геранилгеранил редуктаза; ГГДФ ‒ геранилгеранилдифосфат; НАДФ(H) ‒ окисленный и восстановленный никотин-амидадениндинуклеотидфосфат; ФФн ‒ неорганический дифосфат (по: Averina et al., 2019; с изменениями).
Фотосинтетический аппарат цианобактерий с конститутивным образованием хлорофилла d
Цианобактерии образуют длинноволновые хлорофиллы и включают их в состав фотосинтетического аппарата согласно альтернативным стратегиям: конститутивному синтезу Хл d и индуцированному синтезу Хл f или Хл f/Хл d.
Соотношение Хл a : Хл d в клетках A. marina (в среднем 0.05) тем выше, чем ярче свет и меньше парциальное давление кислорода (Mimuro et al., 2004; Lin et al., 2013). Феофитинизация и дефитилирование Хл d усиливаются в темноте и при аноксии (Tsuzuki et al., 2022). Таким образом, метаболизм Хл d находится, как минимум, под двойным экологическим контролем.
Каротиноиды представлены преимущественно зеаксантином, а также α-каротином, который образуется вместо обычного у цианобактерий β‑каротина (Miyashita et al., 1997).
Состав гидрофильных пигментов штаммоспецифичен. Вначале было показано, что у A. marina MBIC 11017 имеются фикоцианин (ФЦ) и аллофикоцианин (АФЦ) (Hu et al., 1999), и что гены субъединиц CpcA–G находятся на плазмиде pREB3, а гены субъединиц ApcA и ApcB ‒ на хромосоме (Swingley et al., 2008). Новые исследования показали, что у данного штамма АФЦ присутствует в следовом количестве, и поэтому роль терминального переносчика энергии может выполнять ФЦ (Bar-Zvi et al., 2018). Кроме того, показано, что ходе эволюции анцестральные гены ФЦ были утрачены, а затем их отдаленные гомологи были приобретены путем горизонтального переноса. Гены apcA и apcB, в свою очередь, являются отдаленными гомологами генов собственно АФЦ, однако их происхождение неизвестно (Ulrich et al., 2021).
В отличие от штамма MBIC 11017, у штаммов CCMEE5410 и HICR111A отсутствуют ФЦ и АФЦ (Chen et al., 2009; Mohr et al., 2010; Miller et al., 2011).
Cветособирающий комплекс. В фотосинтетическом аппарате Acaryochloris spp. молекулы Хл d выполняют двоякую роль: хлорофилла ССК и хлорофилла реакционных центров РЦ I и РЦ II (Itoh et al., 2007). В состав CCK, общего для ФС I и ФС II (Schiller et al., 1997; Chen et al., 2005c, 2005d), входят белки суперсемейства CBP (сокр. англ. Chlorophyll Binding Protein). Эти шестидоменные белки могут связывать Хл a, Хл a2, Хл b, Хл b2 или Хл d (Chen et al., 2008; Пиневич и соавт., 2010). Они сходны с белками семейства Cp43/IsiA (CP43, или PsbC, входит в антенну РЦ II; IsiA, или CP43′ ‒ это индуцированная железным стрессом субъединица ФС II). Важно, что белки CBP отличаются от трехдоменных белков суперсемейства CAB (сокр. англ. chlorophyll a/b protein) в хлоропластах (La Roche et al., 1996).
Группа из 18 молекул белка CBP окружает тройной комплекс ФС I, а 8 молекул фланкируют 4 комплекса ФС II (Chen et al., 2005a, 2005b). При железном стрессе вместо белка CBP-A синтезируется белок CBP-C; транскрипция его гена индуцируется и при ослаблении освещения (Chen et al., 2005a; Swingley et al., 2005).
Как отмечалось в начале раздела, в состав порфиринов ССК A. marina может входить и Mg-ДВП. Однако вопрос о функционировании этого Хл с-подобного пигмента в фотофизических процессах остается открытым (Schliep et al., 2008); более реальной видится его роль как ключевого интермедиата биосинтеза хлорофиллов (рис. 2).
У штаммов A. marina, наряду с Хл d-содержащим ССК, имеется фикобилипротеиновый ССК (Chen et al., 2009). В то время как т.н. “стандартная ФБС” (MacColl, 1998) имеет форму половины диска, этот ССК ‒ цилиндрический и образован стержнями, состоящими из тримеров ФЦ (Niedzwiedzki et al., 2019). Ввиду отсутствия окрашенного “анкерного” линкера ApcE неясно, каким образом такая уникальная ФБС прикреплена к тилакоиду; тем не менее, она эффективно передает энергию РЦ II (Hu et al., 1999). Кроме того, тримеры ФЦ должны быть спектрально гетерогенными, чтобы перенос энергии к основаниям стержней стал однонаправленным. По-видимому, это достигается за счет синтеза изоформных мономеров ФЦ, а также вследствие разнообразия линкерных полипептидов (Bar-Zvi et al., 2018; Niedzwiedzki et al., 2019). Так или иначе, располагая сразу двумя светособирающими комплексами, A. marina имеет существенное преимущество перед другими ДКС-адаптированными цианобактериями (Loughlin et al., 2013).
Реакционные центры. Каркас РЦ I A. marina, гетеродимер PsaA/PsaB на 86% сходен с типовым. Первичным донором (P740) служит специальная пара (англ. special pair) ‒ димер Хл d/Хл d ′ (Хл d ′ это C132 алломер Хл d). Согласно консенсусной модели (рис. 3), первичный донор взаимодействует с первичным акцептором (одной из двух молекул Хл a) и вторичными акцепторами, а также с Хл d в кор-антенне; редокс-потенциал возбужденного первичного донора (P740*) равен − 439 мВ (Hu et al., 1998), т.е. он примерно такой же, как у возбужденного первичного донора (Р700*) в РЦ I большинства цианобактерий (Tomo et al., 2008). В свете последних данных консенсусная модель может быть частично пересмотрена. По-видимому, PЦ I уникален: первичным акцептором (A0) вместо Хл a служит феофитин a. Кроме того, ФC I существует как тример; каждый мономер содержит дополнительную субъединицу Psa27, 70‒77 молекул Хл d, одну молекулу Хл a, две молекулы феофитина d, 12‒13 молекул α-каротина, две молекулы филлохинона, три Fe-S кластера, две молекулы фосфатидилглицерола и одну молекулу моногалактозилдиглицерина (Hamaguchi et al., 2021; Xu et al., 2021; Kimura et al., 2022).
Рис. 3.
Схема фотосистем у цианобактерий с длинноволновыми хлорофиллами. Объяснение в тексте. Вверху слева: ФC I у Acaryochloris marina. Обозначения: A, B – каркасный гетеродимер субъединиц, действующая и недействующая ветви соответственно; Pc – пластоцианин; PA, PB ‒ первичный донор (димер Хл d); A0 (d−1A, d−1B ‒ Хл d; a0A, a0B ‒ Хл a) ‒ первичный акцептор; A1A, A1B ‒ промежуточный акцептор; FX ‒ вторичные акцепторы (FeS-кластеры). Внизу слева: ФС I у Chroococcidiopsis thermalis. Обозначения: как указано выше, за исключением PA, PB (в данном случае это димер Хл a) и A0 (в данном случае d–1A это Хл f вместо Хл d). Вверху справа: ФC II у A. marina (по: Averina et al., 2018; c изменениями). Обозначения: D1, D2 ‒ каркасный димер субъединиц, действующая и недействующая ветви соответственно; PD1, PD2 ‒ первичный донор (гетеродимер Chl a/Chl d); dD1, dD2 ‒ первичный акцептор (Хл d); Фео a ‒ вторичный акцептор (феофитин a); QA, QB ‒ вторичные акцепторы (хиноны); dZ ‒ Хл d антенны реакционного центра; YZ, YD ‒ остатки Tyr; Mn ‒ марганцевый кластер H2O-окисляющего комплекса. Внизу справа: ФС II у Ch. thermalis. Обозначения: как указано выше, за исключением PD1, PD2 (в данном случае это димер Chl a) и dD1 (в данном случае вместо Хл d это может быть Хл f). aZ ‒ Хл a антенны реакционного центра.
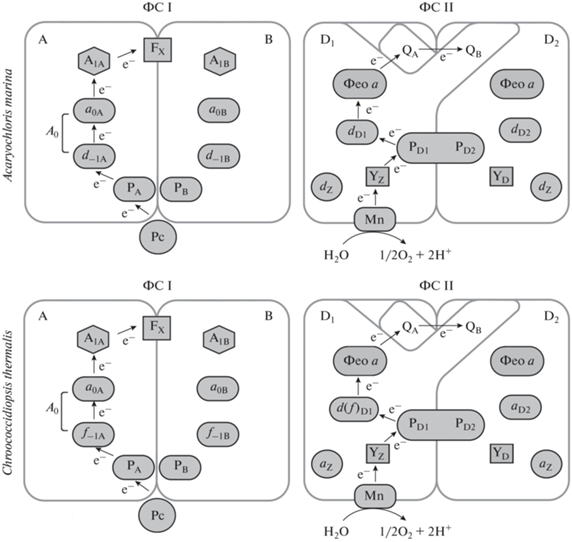
Строение РЦ II до конца не ясно. В отношении первичного донора были выдвинуты две гипотезы. Согласно первой из них, это специальная пара молекул Хл d (Itoh et al., 2007). Сильный контраргумент основан на термодинамических расчетах (Allakhverdiev et al., 2016): энергии, поглощенной ССК, недостаточно для работы четырехтактного механизма фотоокисления воды (т.н. Cock’s clock). Согласно второй гипотезе, это ‒ как обычно у цианобактерий ‒ специальная пара молекул Хл a (P680) (Mimuro et al., 1999). Однако в данном случае пришлось бы допустить, что энергия мигрирует против термодинамического потенциала: ССК содержит Хл d, а тот по сравнению с Хл a поглощает кванты меньшей частоты. В доказательство второй гипотезы были использованы данные измерения флуоресценции клеток при 77 K (Mimuro et al., 1999, 2004), а также результаты биохимического анализа, согласно которым соотношение Хл a : : феофитин a (1 : 1) не изменялось при разных режимах освещения (Mimuro et al., 1999, 2000; Akiyama et al., 2002; Mimuro et al., 2004). Следует отметить, что обе гипотезы были предложены до начала опытов с мембранными препаратами A. marina. Полученные в них результаты, в частности, с помощью метода инфракрасной спектроскопии с преобразованием Фурье (FTIR), указывали на возможность существования P713 ‒ специальной пары молекул Хл d (Tomo et al., 2007); результаты анализа очищенных препаратов ФС II подтверждали это (Itoh et al., 2007). Из термодинамических расчетов, напротив, следовало, что первичный донор ‒ гетеродимер Хл a/Хл d (Renger, Schlodder, 2008). В настоящее время утвердилась консенсусная модель (Allakhverdiev et al., 2016), согласно которой (рис. 3) в РЦ II содержатся две молекулы феофитина a и 6 молекул хлорофилла, в том числе, не менее четырех молекул Хл d. Первичным акцептором, скорее всего, служит молекула Хл d (dD1) на действующей ветви; квазисимметричная ей молекула Хл d (dD2) находится на недействующей ветви. Оставшиеся две молекулы Хл d (dz) выполняют антенную функцию. Вторичным акцептором, скорее всего, является феофитин a. Подводя итог, отметим, что неоднозначность выводов о строении РЦ II у Acaryochloris spp., безусловно, будет устранена по мере расширения круга модельных штаммов и использования более совершенных аналитических методов.
ХЛОРОФИЛЛ f
Chl f ‒ это минорный хлорофилл (∼10% содержания Хл a) у ряда одноклеточных цианобактерий, в частности, Aphanocapsa sp. KC1 (Miyashita et al., 2014), штамма NSW (Behrendt et al., 2015), Synechococcus sp. PCC 7335 (Gan et al., 2015) и Altericista variichlora CALU 1173 (Averina et al., 2021). Он также обнаружен у некоторых нитчатых цианобактерий, например, Halomicronema hongdechloris C2206 (Chen et al., 2012), Chlorogloeopsis fritschii PCC 6912 (Airs et al., 2014), Leptolyngbya sp. JSC-1 (Gan et al., 2014), Chlorogloeopsis sp. PCC 9212 (Gan et al., 2015) и Ch. fritschii CALU 759 (Averina et al., 2018).
Структура и оптические свойства. При относительно небольшом структурном различии Хл f и Хл a (2-формил вместо 2-метила; рис. 1), Q-полоса сильно смещена в длинноволновую область (665 → 698 нм, в 100%-ном ацетоне; см. Averina et al., 2019). Таким образом, Хл f ‒ наименее энергоемкий среди известных хлорофиллов (Chen et al., 2010).
Биосинтез. Хл f ‒ регионально более окисленная молекула, чем Хл a. Теоретически, он может образовываться альтернативными способами (рис. 2). В первом случае окисляется метильная группа в молекуле Хл a; во втором случае окислению подвергается хлорофиллид a (Chen et al., 2010; Miyashita et al., 2014; Ho et al., 2016). Методами обратной генетики и гетерологической экспрессии показано, что Хл f синтазу кодирует ген psbA4, который имеется у всех адаптирующихся к ДКС цианобактерий (Ho et al., 2016). Этот ген ‒ паралог генов семейства psbA (гены ФС II), и хотя в его продукте, белке PsbA4, нет домена для связывания марганцевого кластера Н2O-окисляющего комплекса, в нем присутствуют лиганды для Хл a и консервативный остаток Tyr (Yz) (Gan et al., 2015). Хотя Хл f в следовом количестве образуется даже на белом свету, для адаптивного биосинтеза требуется ДКС, т.е. белок PsbA4 ‒ это фотооксидоредуктаза (Ho et al., 2017b; Shen et al., 2019). Во избежание путаницы, чтобы не смешивать ген Хл f синтазы с геном-паралогом, который кодирует субъединицу PsbA1 (другое обозначение ‒ субъединица D1) в составе РЦ II, было предложено переименовать ген psbA4 в chlF, а белок PsbA4 ‒ в ChlF (Ho et al., 2016). По-видимому, ген chlF является анцестральным геном, и остальные psbA-гены произошли от него путем дупликации и дивергенции (Murray, 2012; Cardona et al., 2015). Иными словами, Хл f ‒ более архаичный признак, чем ФС II (Ho et al., 2016).
Фотосинтетический аппарат цианобактерий с индуцированным образованием длинноволновых хлорофиллов
Проблема энергетического сопряжения между длинноволновыми хлорофиллами и Хл a. На возможность миграции энергии от длинноволновых хлорофиллов к Хл a указывает то, что на перифериях ФС I и ФС II обнаружены пулы молекул Хл f (Itoh et al., 2015). Однако перенос энергии от Хл f к Хл a проблематичен из общетеоретических соображений. Действительно, Хл a получает энергию от пигментов, интенсивно поглощающих видимый свет (Хл b, каротиноидов и фикобилипротеинов), тогда как Хл f не только слабо поглощает свет в этой области, но и усиленно флуоресцирует. К тому же Chl f должен подчиняться правилу Стокса (возбужденное состояние передается пигменту с максимумом поглощения при большей длине волны).
Тем не менее, перенос энергии от Хл f к Хл a ‒ это не работа против термодинамического потенциала (англ. uphill): второе начало термодинамики, скорее всего, соблюдается благодаря сочетанию специфических факторов. К ним относятся: а) долгое пребывание Хл f в возбужденном состоянии; б) низкая энтропия, по статистике Больцмана, системы молекул Хл f; в) близкое соседство молекул Хл f и Хл a; г) особая связь между длинноволновыми хлорофиллами и апопротеинами (Niedzwiedzki et al., 2014; Itoh et al., 2015; Allakhverdiev et al., 2016; Larkum et al., 2018). Функционирование Хл f в качестве переносчика энергии подтверждается результатами спектрометрии H. hongdechloris (Tomo et al., 2014; Akimoto et al., 2015) и теоретическими расчетами с применением методов математического моделирования (Schmitt et al., 2019).
Светособирающий комплекс. По априорным представлениям, Хл f прежде всего антенный пигмент (Chen, Blankenship, 2011; Allakhverdiev et al., 2016; Nürnberg et al., 2018), хотя конкретные данные о составе хлорофиллсодержащего ССК у цианобактерий, индуцированно образующих Хл f или Хл f/Хл d, в настоящее время отсутствуют. Зато известно, как при адаптации к ДКС изменяется структура ФБС. В частности, у Leptolyngbya sp. JSC-1 количество цилиндрических суперкомплексов кора уменьшается с трех до двух (Gan et al., 2014). Из-за изменения состава хромофоров максимум поглощения кора смещается на 40 нм в длинноволновую сторону (обычно он при ~650 нм; Glazer, Bryant, 1975). При адаптации к ДКС у H. hongdechloris вместо пятицилиндрового кора образуется двухцилиндровый кор из субъединиц АФЦ с максимумами поглощения при 653 и 712 нм (Li et al., 2016). У адаптированного к ДКС штамма Synechococcus sp. PCC 7335 имеются “стандартная ФБС” (трехцилиндровый кор; периферические стержни из ФЦ и АФЦ) и “мини-ФБС” (двухцилиндровый кор из аллофикоцианинов ApcB2, Ap-cD2/D3/D5, ApcE2 и ApcF). Максимум поглощения “мини-ФБС” расположен при большей длине волны (711 нм вместо 650 нм), и пик низкотемпературной флуоресценции при 730 нм (Ho et al., 2017a).
Длинноволновые сдвиги “стандартной ФБС” и образование “мини-ФБС” обеспечивают миграцию энергии в направлении АФЦ → Хл f. Кроме того, установлено, что разделение зарядов в РЦ II ускоряется при наличии АФЦ-содержащих длинноволновых антенн, тогда как у остальных известных типов фотосистем увеличение эффективной площади ССК, напротив, замедляет разделение зарядов (Mascoli et al., 2022).
Реакционные центры. Неизвестно, участвует ли Хл f в первичной фотохимии (Allakhverdiev et al., 2016). Результаты опытов с выращенными на ДКС клетками Chroococcidiopsis thermalis теоретически допускают, что Хл f участвует в разделении зарядов в РЦ I и РЦ II (Nürnberg et al., 2018) (рис. 3). Очевидно, что фотохимическая активность специальной пары Хл fХл f (или Хл f/Хл d) зависит от того, будет ли редокс-потенциал возбужденного состояния выше по абсолютной величине, чем у P700* и P680* (Nürnberg et al., 2018).
ФС I, как следует из анализа клеток Ch. thermalis, выращенных на ДКС, содержит 7‒8 молекул Хл f, причем большинство из них входит в кор-антенну (Shen et al., 2019; Gisriel et al., 2020). Недавний анализ ФС I Fischerella thermalis с помощью фемтосекундной спектроскопии (FTIR) показывает, что у этой цианобактерии длинноволновые хлорофиллы ‒ исключительно антенные пигменты (Cherepanov et al., 2020). Однако предыдущее изучение F. thermalis и Ch. thermalis этим же методом (Nürnberg et al., 2018; Hastings et al., 2019) позволило считать, что, хотя длинноволновые хлорофиллы и не выполняют функцию первичного донора (PA/PB), первичным акцептором (f–1А и/или f–1B) все же может быть Хл f (рис. 3).
ФС II в клетках Ch. thermalis, выращенных на ДКС, содержит в основном Хл a (до 30 молекул), а также 4 молекулы Хл f и одну молекулу Хл d. Только одна молекула длинноволнового хлорофилла участвует в переносе электронов; она, видимо, входит в специальную пару (Nürnberg et al., 2018). По уточненным данным, такая молекула находится в положении-D1 (Nürnberg et al., 2018; Judd et al., 2020), а не в положении-PD1, как считали ранее (см. Gorka et al., 2021). Альтернативным членом специальной пары может быть длинноволновый хлорофилл в положении-РD2 (рис. 3). Иными словами, специальной парой в ФС II считается, хотя и не окончательно, гетеродимер Хл а/Хл f или гетеродимер Хл а/Хл d (Judd et al., 2020). Недавно проведенное крио-ЭМ моделирование ФС II для ДКС-адаптированных клеток Synechococcus sp. PCC 7335 позволило идентифицировать одну молекулу Хл d в положении-PD1 и четыре молекулы Хл f в кор-антенне (Gisriel et al., 2021). Согласно филогенетической реконструкции, адаптированная к ДКС анцестральная ФС II содержала в цепи переносчиков одну молекулу Хл d, а также две молекулы Хл f и три молекулы Хл a в составе антенных субъединиц, причем современная структура могла сформироваться разными способами (Gisriel et al., 2022).
Благодаря внедрению в сердцевину ФС II отдельных молекул длинноволнового хлорофилла ДКС-адаптирующиеся цианобактерии эффективнее реагируют на изменение спектрального состава света, чем A. marina (Mascoli et al., 2022).
КЛАСТЕР ФОТОАДАПТАЦИИ К ДАЛЬНЕМУ КРАСНОМУ СВЕТУ (FaRLiP)
Генный кластер FaRLiP (сокр. англ. Far-Red Light Photoacclimation) ответствен за комплекс изменений при фотосинтетической “акклиматизации” к световому режиму, при котором ДКС преобладает над видимым светом (Gan et al., 2014, 2015; Gan, Bryant, 2015). В процессе фотоакклиматизации индуцируется образование Хл f или Хл f/Хл d и перестраиваются ФС I, ФС II и ФБС (Gan et al., 2014; Gan, Bryant, 2015; Gan et al., 2015). По результатам протеомного анализа, несмотря на переход на качественно новый световой режим, паттерн метаболизма в целом сохраняется. Изменение белкового состава избирательно затрагивает фотосинтетический аппарат: образуются апопротеины Хл f-содержащих фотосистем и вместо “стандартной ФБС” ‒ субъединицы длинноволновой (red-shifted) ФБС (Chen et al., 2019).
По данным в свободном доступе, кластер FaRLiP присутствует у 20 штаммов цианобактерий (Gan et al., 2014, 2015; Trampe, Kühl, 2016; Antonaru et al., 2020). В частности, к ним относятся одноклеточные цианобактерии Ch. thermalis PCC 7203 и Synechococcus sp. PCC 7335, а также нитчатые цианобактерии Calothrix sp. PCC 7507, Chlorogloeopsis sp. PCC 9212 и F. thermalis PCC 7521.
В кластер FaRLiP входит 21 ген. Это, в первую очередь, гены rfpA, rfpB и rfpC, кодирующие белки двухкомпонентной фосфорелейной системы (Zhao et al., 2015). Продукт гена rfpA ‒ фоторецептор RfpA ‒ принадлежит к группе т.н. “knotless” (не имеющих сенсорного PAS-домена) фитохромов. Проводником сигнала служит CheY-подобный белок RfpC, регулятором ответа ‒ белок RfpB, который содержит два CheY-подобных домена, фланкирующих ДНК-связывающий домен. Белок RfpB действует как позитивный регулятор генов кластера FaRLiP (Zhao et al., 2015; Ho et al., 2017b). Мутанты Ch. fritschii PCC 9212, Ch. thermalis PCC 7203 и Synechococcus sp. PCC 7335 по генам rfpA, rfpB и rfpC не образуют Хл f, тогда как Хл d образуется и на белом свету, и на ДКС (Zhao et al., 2015). Регуляторные гены кластера FaRLiP обычно расположены на хромосоме в порядке rfpB‒rfpA‒rfpC (Gan et al., 2015); исключение составляют новые виды A. variichlora (Averina et al., 2021) и Kovacikia minuta (Shen et al., 2022), у которых ген rfpB обособлен от генов rfpA и rfpC (рис. 4).
Рис. 4.
Схематическое изображение генного кластера FaRLiP у представителей разных родов цианобактерий. Обозначения генов из отдельных групп приведены только для ранее описанного вида A. variichlora (Averina et al., 2021).
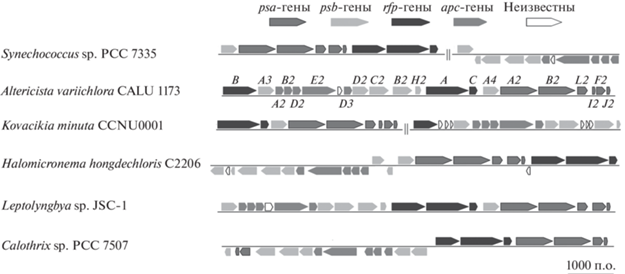
К числу нерегуляторных генов кластера FaRLiP принадлежат паралоги генов субъединиц ФС I ‒ psaA2/B2/F2/I2/J2/L2, а также паралоги генов субъединиц ФС II ‒ psbA3/A4/B2/C2/D2. Продукты этих генов связывают не только Хл a, но и оба длинноволновых хлорофилла (Gan, Bryant, 2015). По результатам протеомного анализа штамма Leptolyngbya sp. JSC-1, каркас РЦ II образуют продукты генов-паралогов ‒ белки PsbA3 и PsbA4 (Gan et al., 2014). Белок PsbA3 содержит полный набор сайтов для лигандирования марганцевого кластера и других фотохимически активных компонентов ФС II (Murray, 2012; Cardona et al., 2015; Gan et al., 2015). По сравнению с ним белок PsbA4 (другое обозначение ‒ srPsbA; сокр. англ. super rogue ‒ “особо резко отличающаяся форма белка PsbA”) настолько модифицирован, что в нем отсутствует сайт для связывания марганцевого кластера (Murray, 2012; Cardona et al., 2015; Gan et al., 2015). Как отмечено, белок PsbA4 дополнительно выполняет функцию Хл f синтазы: при гетерологической экспрессии в клетках Synechococcus sp. PCC 7002 генов psbA4 Ch. fritschii PCC 9212 и F. thermalis PCC 7521 начинается синтез Хл f (Ho et al., 2016; Shen et al., 2019). Иными словами, адаптированная к ДKС фотосистема с гомодимером белка PsbA4 или гетеродимером белков PsbA4/PsbA1 (D1) не только выполняет роль универсального донора электронов, но и напрямую участвует в одной из реакций конструктивного метаболизма (Shen et al., 2019; Trinugroho et al., 2020).
В кластер FaRLiP также входят паралоги генов apcA2/B2/D2/D3/E2, кодирующих аллофикоцианинные субъединицы кора ФБС (Gan et al., 2014; Zhao et al., 2015). При адаптации к ДКС приостанавливается синтез фикоэритрина и фикоэритроцианина ‒ фикобилипротеинов с зеленым (∼550 нм) максимумом поглощения (Gan et al., 2015; Soulier et al., 2020). На белом свету эти пигменты расположены на дистальном конце стержней ФБС; теперь же стержни укорачиваются, обогащаясь ФЦ ‒ рецептором ближнего красного света (∼650 нм). В отличие от аллофикоцианина ApcA1, образующегося на белом свету, аллофикоцианины ApcA2, ApcD2 и ApcD3 содержат дополнительные остатки Cys, которые не связывают хромофоров и не образуют дисульфидных мостиков (Gan et al., 2015; Herrera-Salgado et al., 2018; Bryant et al., 2020). Поскольку у выращенных на ДКС мутантов Synechococcus sp. PCC 7335 по генам apc резко уменьшается содержание Хл d, дополнительные остатки Cys, видимо, участвуют в образовании этого хлорофилла (Bryant et al., 2020), Продукт еще одного гена-паралога, белок АрсЕ1 (другое обозначение ‒ LCM; сокр. англ. Linker Core-Membrane) прикрепляет кор ФБС к мембране тилакоида; аминокислотная последовательность его N-концевого участка сходна с аминокислотной последовательностью α-субъединицы аллофикоцианина ApcA1. Наконец, хотя белок АрсЕ2 содержит “карман” для хромофора (а.о. номеров 180‒230), фикоцианобилин связывается нековалентно, и максимум поглощения перемещается в длинноволновую область (Gan et al., 2015; Miao et al., 2016; Ho et al., 2017a).
ЦИАНОБАКТЕРИИ С КОНСТИТУТИВНЫМ ОБРАЗОВАНИЕМ ХЛОРОФИЛЛА d
Штамм A. marina MBIC 11017, первый из обнаруженных Хл d-содержащих организмов, был выделен из колонии асцидий Lissoclinum patella в западной части Тихого океана (Miyashita et al., 1996). В данном случае этот тип хлорофилла составлял большинство молекул хлорофилла: на Хл a в зависимости от светового режима приходилось только 1‒10% (Miyashita et al., 1997; Mimuro et al., 2004; Lin et al., 2013). Штаммы, образующие Хл d, также выделены из симбиотических ассоциаций A. marina с другими асцидиями ‒ Diplosoma spp. (Kühl et al., 2005), L. fragile (López-Legentil et al., 2011) и Cystodytes dellechiajei (Martinez-Garcia et al., 2011). Наряду с типовым штаммом MBIC 110017, вид A. marina и родственные виды представлены эпифитными, эпизойными и эндозойными штаммами из низких широт Мирового океана: Awaji-1 (Murakami et al., 2004), CCMEE 5410 (Miller et al., 2005), MBIC 10697 (Swingley et al., 2005), HICR 111A (Mohr et al., 2010), MPGRS1 (Larkum et al., 2012), CRS (Behrendt et al., 2013) и Ssball 1 (Lin et al., 2013), а также эпилитными и эндолитными штаммами разного географического происхождения (см. ниже).
Морфология и ультраструктура цианобактерий рода Acaryochloris
Штаммы Acaryochloris spp. совместно кластеризуются на дендрограмме (рис. 5). Их морфологическое разнообразие невысоко: например, в отличие от одиночных клеток типового штамма A. marina MBIC 11017 (Miyashita et al., 1996), клетки Acaryochloris sp. HICR111A образуют неупорядоченные агрегаты (Mohr et al., 2010). Ультраструктура ламеллярной системы однообразна: в частности, тилакоиды Acaryochloris sp. CCMEE 5410 и Acaryochloris sp. MPGRS1 лежат параллельными рядами вдоль цитоплазматической мембраны (Miller et al., 2005; Larkum et al., 2012). Исключением служит вид-кандидат Сandidatus Acaryochloris bahamensis, у которого зона флуоресценции фикобилипротеинов (при 640‒670 нм) не совпадает с зоной флуоресценции Хл d (при ∼750 нм) (Lopez-Legentil et al., 2011). По-видимому, наблюдаемая анизотропия объясняется неравномерным распределением “мини-ФБС” по ламеллярной системе, что, в свою очередь, может быть следствием векторной ориентации симбионта в теле асцидии L. fragile.
Рис. 5.
Филогенетическое древо цианобактерий, образующих длинноволновые хлорофиллы, основанное на сравнении (методом Maximum Likelihood) последовательностей гена 16S рРНК. В узлах ветвления – достоверность (bootstrap) для 1000 альтернативных деревьев; значения <50% не показаны. Обозначения: белые треугольники – штаммы c конститутивным образованием Хл d; белые круги – штаммы с индуцированным образованием Хл f или Хл f/Xл d; черные круги – полученные авторами штаммы с индуцированным образованием Хл f/Xл d (не опубликованы за исключением A. variichlora CALU 1173; см.: Averina et al., 2021).
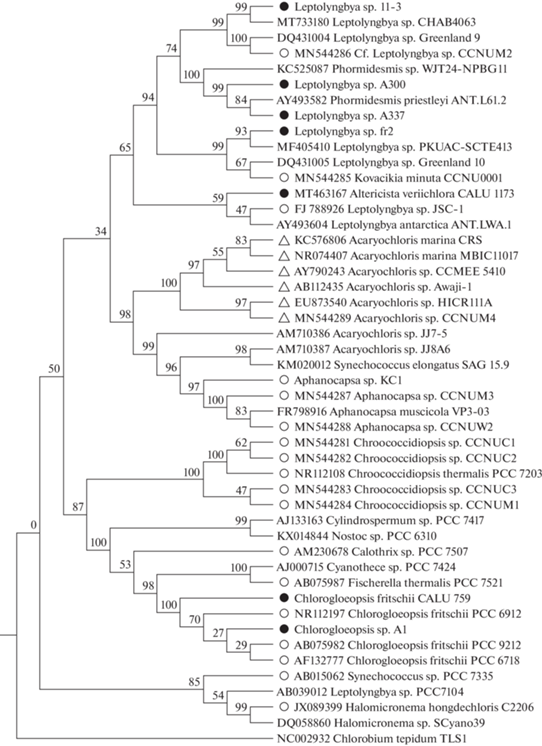
Эколого-географическое разнообразие штаммов Acaryochloris spp.
Эти цианобактерии приурочены к бедным видимым светом морским местообитаниям и континентальным водоемам. Они образуют биопленки и микробные маты (в том числе, строматолиты) или участвуют в симбиотических ассоциациях с водорослями и беспозвоночными (Murakami et al., 2004; Kühl et al., 2005; Miller et al., 2005; Ohkubo et al., 2006; Mohr et al., 2010; Behrendt et al., 2011; Martinez-Garcia et al., 2011; Li et al., 2013).
Обитатели коралловых рифов. По данным метагеномного анализа, штаммы р. Acaryochloris часто встречаются в биопленках на коралловых рифах. В таких местообитаниях филотипы, включающие последовательности 16S рДНК Aсaryochloris spp., насчитывают 5% цианобактериальных и 1.8% суммы бактериальных последовательностей (Behrendt et al., 2011). Специфической нишей для Acaryochloris spp., в частности, для штамма HICR111A, служат останки кораллов на Большом Барьерном рифе, о чем свидетельствуют морфологические, спектроскопические и молекулярно-генетические данные (Mohr et al., 2010; Behrendt et al., 2011, 2013).
Симбионты асцидий. Большинство Хл d-содержащих симбионтов асцидий принадлежат к числу некультивируемых бактерий (Ohkubo, Miyashita, 2012). По результатам ПЦР с использованием праймеров для амплификации гена 16S рРНК представителей р. Aсaryochloris, симбионты асцидий Didemnum spp., Lissoclinum patella, L. punctatum и L. timorense образуют 14 филотипов (Ohkubo, Miyashita, 2012). Хл d-содержащие симбионты колониальных асцидий Diplosoma similis и D. virens скапливаются в базальном отделе тела хозяина (Kühl et al., 2005). Микроколонии цианобактерий с такими же спектральными характеристиками, как у штаммов р. Aсaryochloris, прикрепляются к тунике средиземноморской асцидии Cystodytes dellechiajei (Martinez-Garcia et al., 2011). Хл d-содержащие штаммы размножаются в тунике взрослых асцидий L. fragile и личинок, что указывает на вертикальный путь наследования микросимбионта. С учетом <5%-ного сходства последовательностей 16S рДНК этих цианобактерий и A. marina MBIC 11017 они отнесены к виду-кандидату Сandidatus Acaryochloris bahamiensis (López-Legentil et al., 2011).
Эпифиты. Хл d-содержащие эпифиты обнаружены с помощью спектроскопии и флуоресцентного анализа у морских красных водорослей Ahnfeltiopsis flabelliformis, Callophyllis japonica и Carpopeltis prolifera (Murakami et al., 2004). По результатам секвенирования 16S рДНК, сходные с A. marina штаммы ассоциированы с красными водорослями Caulacanthus ustulatus, Chondria crassicaulis, Ch. ocellatus, Gloiopeltis furcata и Grateloupia lanceolata, а также с зеленой водорослью Ulva pertusa и бурой водорослью Undaria pinatifida (Ohkubo et al., 2006). Штамм MPGRS1 выделен в Юго-Восточной Австралии с таллома красной водоросли Gelidium caulacantheum, растущей на пневматофоре мангрового дерева Avicennia marina. Желтоватая взвесь в воде сильно поглощает видимый свет, что способствует избирательному росту цианобактерий, образующих Хл d (Larkum et al., 2012). Отдельный экотип выявлен в субтропических лесах Центрального Китая; наряду со штаммом Acaryochloris sp. CCNUM4, в него входят штаммы, имеющие близких родственников среди морских цианобактерий (Zhang et al., 2019). Упомянутые штаммы ассоциированы со мхами на известняковых породах и макрофитами в прудах и ручьях; в таких затененных нишах обычно много ДКС на фоне остаточного белого света. По последним данным, у штаммов Acaryochloris sp., выделенных в калифорнийской приливно-отливной зоне с талломов красных водрослей Chondracanthus sp. и Neogastroclonium sp. и бурой водоросли Desmarestia sp., содержание Хл d составляет 99% всего хлорофилла (Kiang et al., 2022). По результатам спектрометрического анализа in situ, это самый коротковолновый из хлорофиллов, известных у р. Acaryochloris (Q-полоса при 704‒705 нм).
Эпилиты и эндолиты. Штамм CCMEE 5410 выделен из донного микробного мата в умеренно галофильном оз. Солт-Лейк в Южной Калифорнии (Miller et al., 2005). Последовательности 16S рДНК у представителей р. Aсaryochloris встречаются в ампликонных библиотеках материала с каменистого дна водоемов умеренных и высоких широт. По данным метагеномного анализа, цианобактерии, сходные со штаммами р. Aсaryochloris, участвуют в микробных сообществах на пирамидах Майя (McNamara et al., 2006), на гранитных скалах Антарктиды (de los Rios et al., 2007) и на ископаемых строматолитах Боливийских Анд (Fleming, Prufert-Bebout, 2010).
Эндолиты, близкие штаммам р. Aсaryochloris, ассоциированы с известковыми кораллиновыми водорослями. Они охарактеризованы с помощью TaqMan-ПЦР в материале из Красного моря и с Большого Барьерного рифа, а также собранного на морском побережье в Испании и Хорватии; численность этих цианобактерий варьирует от 40 клеток до 1.51 × 103 клеток на 1 мг образца (Behrendt et al., 2011, 2014).
ЦИАНОБАКТЕРИИ C ИНДУЦИРОВАННЫМ ОБРАЗОВАНИЕМ ДЛИННОВОЛНОВЫХ ХЛОРОФИЛЛОВ
Как указано, в отличие от штаммов р. Acaryochloris, у которых Хл d присутствует конститутивно, некоторые цианобактерии способны индуцированно образовывать либо один Хл f, либо Хл f одновременно с Хл d (штаммы с индуцированным синтезом одного Хл d неизвестны).
Такие объекты встречаются в морских и пресноводных местообитаниях (Akutsu et al., 2011; Averina et al., 2018) и термальных источниках (Gan et al., 2014, 2015), на заболоченной почве (Airs et al., 2014; Gan et al., 2015) и в карстовых пещерах (Behrendt et al. 2015). В основном они образуют биопленки и микробные маты, в том числе строматолиты (Chen et al., 2010; Trampe, Kühl, 2016).
Штаммы с индуцированным синтезом хлорофилла f
Культивируемые представители этой группы немногочисленны. Первый из них, штамм C2206, был выделен из строматолита в австралийском заливе Шарк-Бей (Chen et al., 2010). Клетки размером 0.6‒0.8 × 1.0‒1.3 мкм (близко к минимуму для цианобактерий; см.: Chisholm et al., 1992) образуют неветвящиеся трихомы с чехлом толщиной 100‒200 нм. На белом свету Хл f не образуется; в адаптированных к ДКС клетках он составляет 12.5% количества Хл a. С помощью полифазного подхода эта цианобактерия описана как новый вид ‒ H. hongdechloris (Chen et al., 2012).
Клетки штамма Aphanocapsa sp. KC1 из озера Бива (Akutsu et al., 2011), размер которых 1.3‒2.0 × × 1.3‒3.0 мкм, образуют неупорядоченные скопления; при двухнедельном росте на ДКС содержание Хл f равно 8% Хл a (Miyashita et al., 2014). Образующие Хл f штаммы Aphanocapsa muscicola VP3-03 и 5N-04, а также штаммы Acaryochloris sp. JJ8A6 и JJ7-5, кластеризуются вместе с Хл d-cодержащими штаммами, однако они не синтезируют этот пигмент (Miyashita et al., 2014). Штаммы, сходные с KC1, выделены из биопленок в австралийских карстовых пещерах Дженолан (Behrendt et al., 2015).
При проведении скрининга во влажных субтропических лесах Центрального Китая были обнаружены образующие Хл f одноклеточные цианобактерии из родов Aphanocapsa и Chroococcidiopsis, а также нитчатые цианобактерии, принадлежащие к двум ранее неизвестным родам сем. Leptolyngbyaceae (Zhang et al., 2019).
Штаммы с индуцированным образованием хлорофиллов f и d
Такие штаммы, в частности, одноклеточные цианобактерии Chroococcidiopsis thermalis PCC 7203 и Synechococcus sp. PCC 7335, выделены из образцов почвы около немецкого г. Грайфсвальда и с раковины моллюска в приливно-отливной зоне у мексиканского курортного города Пуэрто-Пеньяско (Gan et al., 2015). Нитчатая цианобактерия Calothrix sp. PCC 7507 изолирована из сфагнового болота в окрестностях швейцарского г. Кастаниенбаума (Gan et al., 2015). Два штамма нитчатых цианобактерий р. Chlorogloeopsis ‒ Ch. fritschii PCC 6912 и Chlorogloeopsis sp. PCC 9212 ‒ выделены из образцов воды на рисовом поле в районе индийского города Аллахабада и из термального источника близь испанского города Оренсе (Airs et al., 2014; Gan et al., 2015). Еще два нитчатых штамма ‒ Leptolyngbya sp. JSC-1 и F. thermalis PCC 7521 ‒ выделены из плавучего микробного мата в термальном источнике на территории кемпинга Ладьюк и из термального источника в карстовой воронке Мамонт II, оба на территории Йеллоустонского национального парка США (Gan et al., 2014, 2015). Шестой нитчатый штамм, Leptolyngbya sp. CCM4, выделен из строматолита около мексиканского г. Куатросьенегас (Gómez-Lojero et al., 2018). Наконец, путем ретроспективного скрининга у штамма Synechocystis sp. CALU 1173 из коллекции СПбГУ обнаружена способность к индуцированному образованию Хл d и Хл f, и на основе полифазного подхода он диагностирован как типовой штамм нового вида A. variichlora (Averina et al., 2021).
ФИЛОГЕНИЯ И ТАКСОНОМИЯ ЦИАНОБАКТЕРИЙ C ДЛИННОВОЛНОВЫМИ ХЛОРОФИЛЛАМИ
Филогенетические взаимоотношения штаммов, образующих длинноволновые хлорофиллы. Штаммы Acaryochloris spp. объединяются в компактный филогенетический кластер (рис. 5). Из этого следует, что конститутивный синтез Хл d можно рассматривать как позднее эволюционное приобретение (Li, Chen, 2015). Однако высокое сходство с 16S рДНК типового штамма A. marina MBIC 11017 не всегда коррелирует со способностью синтезировать Хл d. В частности, штамм RCC1774 не образует Хл d, но у него имеется Хл b; в частности, это позволило в рамках полифазного подхода описать новый вид ‒ A. thomasi (Partensky et al., 2018). Штаммы-деструкторы нефти KUAC 3056 и KUAC 3106, выделенные из Персидского залива, также не образуют Хл d (Al-Bader et al., 2013). С учетом степени сходства по 16S рДНК с A. marina MBIC 11017, эти штаммы можно было бы отнести к новому виду р. Acaryochloris.
В отличие от компактно кластеризующихся штаммов с конститутивным образованием Хл d (рис. 5), штаммы с индуцированным синтезом длинноволновых хлорофиллов сильно дивергируют (Сhen et al., 2012; Airs et al., 2014; Gan et al., 2014; Miyashita et al., 2014; Behrendt et al., 2015; Gan et al., 2015). Соответственно, данную стратегию можно считать более архаичной, хотя и не исключается часто происходивший горизонтальный перенос всего кластера FaRLiP (Gan et al., 2015).
Хлорофиллы как таксономический признак. Примеры, когда тип хлорофилла используется как таксономический признак, особенно когда этот признак отражен в номенклатуре, довольно редки. Хлорофиллам Хл b или Хл b2 придавалось центральное значение в диагнозах цианобактерий-прохлорофитов Prochloron (Lewin, 1976), Prochlorothrix (Burger-Wiersma et al., 1989) и Prochlorococcus (Chisholm et al., 1992). Повторяющийся элемент родовых имен “-chloro-” вначале указывал на присутствие хлорофилла b, а также на гипотетическую роль предка (элемент “Pro-”) зеленых водорослей Chlorophyceae (см.: Lewin, 1976), впоследствии опровергнутую (см.: Пиневич и соавт., 2010).
Конститутивное образование Хл d ‒ признак р. Acaryochloris, в котором три вида ‒ типовой A. marina (Miyashita et al., 2003), вид-кандидат Candidatus A. bahamiensis (Lopez-Legentil et al., 2011) и описанный по правилам Ботанического Кодекса новый вид A. thomasi (Partensky et al., 2018). На наш взгляд, родовое имя выбрано неудачно: так можно назвать любую бактерию (“Acaryo-”), обладающую хлорофиллом (“-chloris”).
Цианобактерии с индуцированным образованием длинноволновых хлорофиллов распределены по морфологическим Субсекциям I‒V, или условным эквивалентам ботанических порядков, описанным в руководстве Берги (см.: Castenholz, 2015). В частности, вид H. hongdechloris принадлежит к Субсекции III. В данном случае видовой эпитет буквально означает “c красным хлорофиллом” (кит. hong-de ‒ красного цвета), хотя имеется в виду Хл f с максимумом поглощения в ДКС (Сhen et al., 2012). Наконец, видовой эпитет цианобактерии A. variichlora (Субсекция I) указывает на образование разных (“varii-”) хлорофиллов (“-chlora”), в данном случае ‒ Хл a, Хл f и Хл d (Averina et al., 2021).
ЗАКЛЮЧЕНИЕ
Успехи в изучении длинноволновых хлорофиллов и фотосинтетической адаптации цианобактерий к ДКС достигнуты благодаря использованию комплекса современных аналитических методов (особенно биоинформатики) в сочетании с традиционными поисками и описанием ранее неизвестных объектов. Полученные данные дополняют представления о механизмах фототрофии, разнообразии, распространении и экологии фототрофных бактерий. На основе фундаментальных разработок появилась перспектива привлечь дополнительный ресурс световой энергии к технологиям промышленного фотосинтеза. При этом несколько важных задач решены лишь частично: 1) конкретика биосинтеза Хл d и Хл f; 2) структура реакционных центров и ССК, содержащих эти хлорофиллы; 3) распространение и вариабельность кластера FaRLiP; 4) филогения цианобактерий с длинноволновыми хлорофиллами. Растущий интерес к феномену фотосинтетической адаптации цианобактерий к ДКС служит залогом новых открытий и обобщений.
Список литературы
Пиневич А.В., Аверина С.Г., Величко Н.В. Очерки биологии прохлорофитов. С.-Петербург: Изд-во С.-Петерб. ун-та, 2010. 198 с.
Airs R.L., Temperton B., Sambles C., Farnham G., Skill S.C., Llewellyn C.A. Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near-infrared radiation // FEBS Lett. 2014. V. 588. P. 3770‒3777.
Akimoto S., Shinoda T., Chen M., Allakhverdiev S.I., Tomo T. Energy transfer in the chlorophyll f-containing cyanobacterium, Halomicronema hongdechloris, analyzed by time-resolved fluorescence spectroscopies // Photosynth. Res. 2015. V. 125. P. 115–122.
Akiyama M., Miyashita H., Kise H., Watanabe T., Mimuro M., Miyachi S., Kobayashi, M. Quest for minor but key chlorophyll molecules in photosynthetic reaction centers – unusual pigment composition in the reaction centers of the chlorophyll d dominated cyanobacterium Acaryochloris marina // Photosynth. Res. 2002. V. 74. P. 97–107.
Akutsu S., Fujinuma D., Furukawa H., Watanabe T., Ohnishi-Kameyama M., Ono S., Ohkubo S., Miyashita H., Kobayashi M. Pigment analysis of a chlorophyll f-containing cyanobacterium strain KC1 isolated from Lake Biwa // Photomed. Photobiol. 2011. V. 33. P. 35–40.
Al-Bader D., Eliyas M., Rayan R., Radwan S. Subsurface associations of Acaryochloris-related picocyanobacteria with oil-utilizing bacteria in the Arabian Gulf water body: promising consortia in oil sediment bioremediation // Microb. Ecol. 2013. V. 65. P. 555–565.
Allakhverdiev S.I., Kreslavski V.D., Zharmukhamedov S.K., Voloshin R.A., Korol’kova D.V., Tomo T., Shen J.R. Chlorophylls d and f and their role in primary photosynthetic processes of cyanobacteria // Biochemistry (Moscow). 2016. V. 81. P. 201–212.
Allen J.F. Protein phosphorylation in regulation of photosynthesis // Biochim. Biophys. Acta. 1992. V. 1098. P. 275‒335.
Amesz J., Neerken S. Excitation energy trapping in anoxygenic photosynthetic bacteria // Photosynth. Res. 2002. V. 73. P. 73‒81.
Antonaru L.A., Cardona T., Larkum A.W.D., Nürnberg D.J. Global distribution of a chlorophyll f cyanobacterial marker // ISME J. 2020. V. 14. P. 2275–2287. https://doi.org/10.1038/s41396-020-0670-y
Averina S., Polyakova E., Senatskaya E., Pinevich A. A new cyanobacterial genus Altericista and three species Altericista lacusladogae sp. nov., Altericista violacea sp. nov., and Altericista variichlora sp. nov., described using a polyphasic approach // J. Phycol. 2021. V. 57. P. 1517‒1529.
Averina S.G., Velichko N.V., Pinevich A.A., Senatskaya E.V., Pinevich A.V. 2019. Non-a chlorophylls in cyanobacteria // Photosynthetica. 2019. V. 57. P. 1109‒1118.
Averina S., Velichko N., Senatskaya E., Pinevich A. Far-red photoadaptations in aquatic cyanobacteria // Hydrobiologia. 2018. V. 813. P. 1‒17.
Badshah S.L., Mabkhot Y., Al-Showiman S.S. Photosynthesis at the far-red region of the spectrum in Acaryochloris marina // Badshah and Mabkhot Biol. Res. 2017. V. 50. Art. 16. https://doi.org/10.1186/s40659-017-0120-0
Bar-Zvi S., Lahav A., Harris D., Niedzwiedzki D.M., Blankenship R.E., Adir N. Structural heterogeneity leads to functional homogeneity in A. marina phycocyanin // Biochim. Biophys. Acta Bioenerg. 2018. V. 1859. P. 544‒553.
Bauer C.E., Bollivar D.W., Suzuki J.Y. Genetic analyses of photopigment biosynthesis in eubacteria: a guiding light for algae and plants // J. Bacteriol. 1993. V. 175. P. 3919‒3925.
Behrendt L., Brejnrod A.S., Schliep M., Sørensen S.J., Larkum A.W.D., Kühl M. Chlorophyll f-driven photosynthesis in a cavernous cyanobacterium // ISME J. 2015. V. 9. P. 2108–2111.
Behrendt L., Larkum A.W.D., Norman A., Qvortrup R., Chen M., Ralph P., Sørensen S.J., Trampe E., Kühl M. Endolithic chlorophyll d-containing phototrophs // ISME J. 2011. V. 5. P. 1072–1076.
Behrendt L., Nielsen J.L., Sørensen S.J., Larkum A.W.D., Winther J.R., Kühl M. Rapid TaqMan-based quantification of chlorophyll d-containing cyanobacteria in the genus Acaryochloris // Appl. Environ. Microbiol. 2014. V. 80. P. 3244–3249.
Behrendt L., Staal M., Cristescu S.M., Harren F.J.M., Schliep M., Larkum A.W.D., Kühl M. Reactive oxygen production induced by near-infrared radiation in three strains of the Chl d-containing cyanobacterium Acaryochloris marina // F1000Research. 2013. V. 2. Art. 44. https://doi.org/10.12688/f1000research.2-44.v.2
Blankenship R.E., Chen M. Spectral expansion and antenna reduction can enhance photosynthesis for energy production // Curr. Opin. Chem. Biol. 2013. V. 17. P. 457‒461.
Bryant D.A., Garcia-Costas A.M., Maresca J.A., Chew A.G.M., Klatt C.G., Bateson M.M., Tallon L.J., Hostetler J., Nelson W.C., Heidelberg J.F., Ward D.W. Candidatus Chloracidobacterium thermofilum: an aerobic phototrophic acidobacterium // Science. 2007. V. 317. P. 523‒526.
Bryant D.A., Shen G., Turner G.M., Soulier N., Laremore T.N., Ho M.-Y. Far-red light allophycocyanin subunits play a role in chlorophyll d accumulation in far-red light // Photosynth. Res. 2020. V. 143. P. 81–95.
Burger-Wiersma T., Stal L., Mur L.R. Prochlorothrix hollandica gen. nov., sp. nov., a filamentous oxygenic photoautotrophic prokaryote containing chlorophylls a and b: assignment to Prochlorotrichaceae fam. nov. and order Prochlorotrichales Florenzano, Balloni, and Materassi 1986, with emendation of the ordinal description // Int. J. Syst. Evol. Microbiol. 1989. V. 39. P. 250‒257.
Cardona T., Murray J.W., Rutherford A.W. Origin and evolution of water oxidation before the last common ancestor of the cyanobacteria // Mol. Biol. Evol. 2015. V. 32. P. 1310‒1328.
Castenholz R.W. General characteristics of the cyanobacteria // Bergey’s Manual of Systematics of Archaea and Bacteria / Eds. DeVos P. et al. Hobolken, NJ: Wiley and Sons, Inc., 2015. https://doi.org/10.1002/9781118960608.cbm00019
Chen M., Bibby T.S., Nield J., Larkum A.W.D., Barber J. Iron deficiency induces a chlorophyll d-binding Pcb antenna system around photosystem I in Acaryochloris marina // Biochim. Biophys. Acta. 2005a. V. 1708. P. 367–374.
Chen M., Bibby T.S., Nield J., Larkum A.W.D., Barber J. Structure of a large photosystem II supercomplex from Acaryochloris marina // FEBS Letters. 2005b. V. 579. P. 306–1310.
Chen M., Blankenship R.E. Expanding the solar spectrum used by photosynthesis // Trends Plant Sci. 2011. V. 16. P. 427‒431.
Chen M., Floetenmeyer M., Bibby T. Supramolecular organization of phycobiliproteins in the chlorophyll d-containing cyanobacterium Acaryochloris marina // FEBS Lett. 2009. V. 583. P. 2535‒2539.
Chen M., Hernandez-Prieto M.A., Loughlin P.C., Li Y., Willows R.D. Genome and proteome of the chlorophyll f-producing cyanobacterium Halomicronema hongdechloris: adaptive proteomic shifts under different light conditions // Genomics. 2019. V. 20. Art. 207. https://doi.org/10/1186/s12864-019-5587-3
Chen M., Hiller R.G., Howe C.J., Larkum A.W.D. Unique origin and lateral transfer of prokaryotic chlorophyll-b and chlorophyll-d light-harvesting systems // Mol. Biol. Evol. 2005c. V. 22. P. 21–28.
Chen M., Li Y., Birch D., Willows R.D. A cyanobacterium that contains chlorophyll f ‒ a red-absorbing photopigment // FEBS Lett. 2012. V. 586. P. 3249–3254.
Chen M., Schliep M., Willows R.D., Cai Z.L., Neilan B.A., Scheer H. A red-shifted chlorophyll // Science. 2010. V. 329. P. 1318–1319.
Chen M., Telfer A., Lin S., Pascal A., Larkum A.W.D., Barber J., Blankenship R.E. The nature of the photosystem II reaction centre in the chlorophyll d-containing prokaryote, Acaryochloris marina // Photochem. Photobiol. Sci. 2005d. V. 4. P. 1060–1064.
Chen M., Zhang Y., Blankenship R.E. Nomenclature for membrane bound light harvesting complexes of cyanobacteria // Photosynth. Res. 2008. V. 95. P. 147–154.
Cherepanov D.A., Shelaev I.V., Gostev F.E., Aybush A.V., Mamedov M.D., Shen G., Nadtochenko V.A., Bryant D.A., Semenov A.Y., Golbeck J.H. Evidence that chlorophyll f functions solely as an antenna pigment in far-red-light photosystem I from Fischerella thermalis PCC 7521 // Biochim. Biophys. Acta Bioenerg. 2020. V. 1861. Art. 148184. https://doi.org/10.1016/j.bbabio.2020.148184
Chisholm S.W., Frankel S.L., Goericke R., Olson J.R., Palenik B., Waterbury J.B., West-Johnsrud L., Zettler E.R. Prochlorococcus marinus nov. gen., nov. sp.: an oxyphototrophic marine prokaryote containing divinyl chloro-phyll a and b // Arch. Microbiol. 1992. V. 157. P. 297–300.
Deisenhofer J., Michel H., Huber R. The structural basis of photosynthetic light reactions in bacteria // Trends Biochem. Sci. 1985. V. 10. P. 243‒248.
Drews G., Niederman R.A. Membrane biogenesis in anoxygenic photosynthetic prokaryotes // Photosynth. Res. 2002. V. 73. P. 87‒94.
Fleming E.D., Prufert-Bebout L. Characterization of cyanobacterial communities from high-elevation lakes in the Bolivian Andes // J. Geophys. Res. 2010. V. 115. Art. 00D07. https://doi.org/10.1029/2008JG000817
French C.S. The chlorophyll in vivo and in vitro // Encyclopedia of Plant Physiology / Ed. Ruhland W. Berlin: Springer-Verlag. 1960. V. 5. Pt. 1. P. 252–297.
Friedrich T., Schmitt F.J. Red-shifted and red chlorophylls in photosystems: entropy as a driving force for uphill energy transfer // Photosynthesis: Molecular Approaches to Solar Energy Conversion. Advances in Photosynthesis and Respiration / Eds. Shen J.R. et al. Cham: Springer. 2021https://doi.org/10.1007/978-3-030-67407-6_9
Fukusumi T., Matsuda K., Mizoguchi T., Miyatake T., Ito S., Ikeda T., Tamiaki H., Oba T. Non-enzymatic conversion of chlorophyll-a into chlorophyll-d in vitro: a model oxidation pathway for chlorophyll-d biosynthesis // FEBS Lett. 2012. V. 586. P. 2338–2341.
Gan F., Bryant D.A. Adaptive and acclimative responses of cyanobacteria to far-red light // Environ. Microbiol. 2015. V. 17. P. 3450–3465.
Gan F., Shen G., Bryant D. Occurrence of far-red light photoacclimation (FaRLiP) in diverse cyanobacteria // Life (Basel). 2015. V. 5. P. 4–24.
Gan F., Zhang S., Rockwell N.C., Martin S.S., Lagarias J.C., Bryant D.A. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light // Science. 2014. V. 345. P. 1312–1317.
Gisriel C.J., Cardona T., Bryant D.A., Brudvig C.W. Molecular evolution of far-red light-acclimated photosystem II // Microorganisms. 2022. V. 10. Art. 1270. https://doi.org/10.3390/microorganisms10071270
Gisriel C.J., Shen G., Ho M.-Y., Kurashov V., Flesher D.A., Wang J., Armstrong W.H., Golbeck J.H., Gunner M.R., Vinyard D.J., Debus R.J., Brudvig G.W., Bryant D.A. Structure of a monomeric photosystem II core complex from a cyanobacterium acclimated to far-red-light reveals the functions of chlorophylls d and f // J. Biol. Chem. 2021. V. 298. Art. 101424. https://doi.org/10.1016/j.jbc.2021.101424
Gisriel C., Shen G., Kurashov V., Ho M.-Y., Zhang S., Williams D., Golbeck J.H., Fromme P., Bryant D.A. The structure of Photosystem I acclimated to far-red light illuminates an ecologically important acclimation process in photosynthesis // Sci. Adv. 2020. V. 6. Art. aay6415. https://doi.org/10.1126/sciadv.aay6415
Glazer A.N., Bryant D.A. Allophycocyanin B (λmax 671, 618 nm) – a new cyanobacterial phycobiliprotein // Arch. Mikrobiol. 1975. V. 104. P. 15–22.
Gómez-Lojero C., Leyva-Castillo L.E., Herrera-Salgado P., Barrera-Rojas J., Ríos-Castro E., Gutiérrez-Cirlos E.B. Leptolyngbya CCM 4, a cyanobacterium with far-red photoacclimation from Cuatro Ciénegas Basin, México // Photosynthetica. 2018. V. 56. P. 342–353.
Gorka M., Baldansuren A., Malnati A., Gruszecki E., Golbeck J.H., Lakshmi K.V. Shedding light on primary donors in photosynthetic reaction centers // Front. Microbiol. 2021. V. 12. Art. 735666. https://doi.org/10.3389/fmicb.2021.735666
Grossman A.R., Schaefer M.R., Chiang G.G., Collier J.L. The phycobilisome, a light-harvesting complex responsive to environmental conditions // Microbiol. Rev. 1993. V. 57. P. 725‒749.
Hamaguchi T., Kawakami K., Shinzawa-Itoh K., Inoue-Kasino N., Itoh S., Ifuku K., Yamashita E., Maeda K., Yonekura K., Kasino Y. Structure of the far-red light utilizing photosystem I of Acaryochloris marina // Nat. Commun. 2021. V. 12. Art. 2333. https://doi.org/10.1038/s41467-021-22502-8
Hastings H., Makita H., Agarwala N., Rohani L., Shen G., Bryant D.A. Fourier transform visible and infrared difference spectroscopy for the study of P700 in photosystem I from Fischerella thermalis PCC 7521 cells grown under white light and far-red light: Evidence that the A–1 cofactor is chlorophyll f // Biochim. Biophys. Acta – Bioenerg. 2019. V. 1860. P. 452‒460.
Herrera-Salgado P., Leyva-Castillo L.E., Ríos-Castro E., Gómez-Lojero C. Complementary chromatic and far-red photoacclimations in Synechococcus ATCC 29403 (PCC 7335). I: The phycobilisomes, a proteomic approach // Photosynth. Res. 2018. V. 138. P. 39–56.
Ho M.-Y., Bryant D.A. Global transcriptional profiling of the cyanobacerium Chlorogloeopsis fritschii PCC 9212 in far-red light: insights into the regulation of chlorophyll d synthesis // Front. Microbiol. 2019. V. 10. Art. 465. https://doi.org/10.3389/fmicb.2019.00465
Ho M.-Y., Gan F., Shen G., Bryant D.A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II. Characterization of phycobiliproteins produced during acclimation to far-red light // Photosynth. Res. 2017a. V. 131. P. 187‒202.
Ho M.-Y., Gan F., Shen G., Zhao C., Bryant D.A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. I. Regulation of FaRLiP gene expression // Photosynth. Res. 2017b. V. 131. P. 173‒186.
Ho M.-Y., Shen G., Canniffe D.P., Zhao C., Bryant D.A. Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II // Science. 2016. V. 353. Art. aaf9178. https://doi.org/10.1126/science.aaf.9178
Holt A.S. Further evidence of the relation between 2-desvinyl-2-formyl-chlorophyll a and d // Can. J. Bot. 1961. V. 39. P. 327–331.
Hu Q., Marquardt J., Iwasaki I., Miyashita H., Kurano N., Mörschel E., Miyachi S. Molecular structure, localization and function of biliproteins in the chlorophyll a/d containing oxygenic photosynthetic prokaryote Acaryochloris marina // Biochim. Biophys. Acta. 1999. V. 1412. P. 250–261.
Hu Q., Miyashita H., Iwasaki I., Kurano N., Miyachi S., Iwaki M., Itoh S. A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis // Proc. Natl. Acad. Sci. USA. 1998. V. 95. P. 13319–13323.
Hunter C.N., van Grondelle R., Olsen J.D. Photosynthetic antenna proteins: 100 ps before photochemistry starts // Trends Biochem. Sci. 1989. V. 14. P. 72‒76.
Itoh S., Mino H., Itoh K., Shigenaga T., Uzumaki T., Iwaki M. Function of chlorophyll d in reaction centers of photosystems I and II of the oxygenic photosynthesis of Acaryochloris marina // Biochemistry. 2007. V. 46. P. 12473–12481.
Itoh S., Ohno T., Noji T., Yamakawa H., Komatsu H., Wada K., Kobayashi M., Miyashita Y. Harvesting far-red light by chlorophyll f in photosystems I and II of unicellular cyanobacterium strain KC1 // Plant Cell Physiol. 2015. V. 56. P. 2024–2034.
Judd M., Mortona J., Nürnberg D., Fantuzzi A., Rutherford A.W., Purchase R., Cox N., Krausz E. The primary donor of far-red photosystem II: ChlD1 or PD2? // Biochim. Biophys. Acta – Bioenerg. 2020. V. 1861. Art. 1482. https://doi.org/10.1016/j.bbabio.2020.148248
Kashiyama Y., Miyashita H., Ohkubo S., Ogawa N.O., Chikaraishi Y., Takano Y., Suga H., Toyofuku T., Nomaki H., Kitazato H., Nagata T., Ohkouchi K. Evidence for global chlorophyll d // Science. 2008. V. 321. P. 658.
Kiang N.Y., Swingley W.D., Gautam D., Broddrick J.T., Repeta D.J., Stolz J.F., Blankenship R.E., Wolf B.M., Detweiler A.M., Miller K.A., Schladweiler J.J., Lindeman R., Parenteau M.N. Discovery of chlorophyll d: isolation and characterization of a far-red cyanobacterium from the original site of Manning and Strain (1943) at Moss Beach, California // Microorganisms. 2022. V. 10. Art. 819. https://doi.org/10.3390microorganisms10040819
Kimura A., Kitoh-Nishioka H., Aota T., Hamaguchi T., Yonekura K., Kawakami K., Shinzawa-Itoh K., Inoue-Kasino N., Ifuku K., Yamasita E., Kasino Y., Itoh S. Theoretical model of the far-red-light-adapted photosystem I reaction center of cyanobacterium Acaryochloris marina using chlorophyll d and the effect of chlorophyll exchange // J. Phys. Chem. 2022. V. 126. P. 4009‒4021.
Kirk J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed. Cambridge e.a.: Cambridge Univ. Press, 1994. 509 p.
Koizumi H., Itoh Y., Hosoda S., Akiyama M., Hoshino T., Shiraiwa Y., Kobayashi M. Serendipitous discovery of Chl d formation from Chl a with papain // Sci. Technol. Adv. Mater. 2005. V. 6. P. 551–557.
Kühl M., Chen M., Ralph P.J., Schreiber U., Larkum A.W.D. A niche for cyanobacteria containing chlorophyll d // Nature. 2005. V. 433. P. 820.
Larkum A.W.D., Chen M., Li Y., Schliep M., Trampe E., West J., Salih A., Kühl M. A novel epiphytic chlorophyll d-containing cyanobacterium isolated from mangrove-associated red alga // J. Phycol. 2012. V. 48. P. 1320–1327.
Larkum A.W.D., Ritchie R.J., Raven J.A. Living off the Sun: chlorophylls, bacteriochlorophylls and rhodopsins // Photosynthetica. 2018. V. 56. P. 11‒43.
La Roche J., van der Staay G.W.M., Partensky F., Ducret A., Aebersold R., Li R., Golden S.S., Hiller R.G., Wrench P.M., Larkum A.W.D., Green B.R. Independent evolution of the prochlorophyte and green plant chlorophyll a/b light-harvesting proteins // Proc. Natl. Acad. Sci. USA. 1996. V. 93. P. 15244–15248.
Lewin R.A. Prochlorophyta as a proposed new division of algae // Nature. 1976. V. 261. P. 697–698.
Li Y., Chen M. Novel chlorophylls and new directions in photosynthesis research // Funct. Plant Biol. 2015. V. 42. P. 493‒501.
Li Y., Larkum A., Schliep M., Kühl M., Neilan B., Chen M. Newly isolated Chl d-containing cyanobacteria // Photosynthesis Research for Food, Fuel and the Future. Proc. 15th Int. Conf. on Photosynthesis / Eds. Kuang T., Lu C., Zhang L. Springer Science + Business Media B.V., 2013. P. 686‒690.
Li Y., Lin Y., Garvey C.J., Birch D., Corkery R.W., Loughlin P.C., Scheer H., Willows R.D., Chen M. Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris // Biochim. Biophys. Acta. 2016. V. 1857. P. 107–114.
Lin Y., Crossett B., Chen M. Effects of anaerobic conditions on photosynthetic units of Acaryochloris marina // Photosynthesis Research for Food, Fuel and the Future: 15th Int. Conf. on Photosynthesis / Eds. Kuang T., Lu C., Zhang L. Springer Science + Business Media B.V., 2013. P. 121‒124.
López-Legentil S., Song B., Bosch M., Pawlik J.R., Turon X. Cyanobacterial diversity and a new Acaryochloris-like symbiont from Bahamian sea-squirts // PLoS One. 2011. V. 6. Art. e23938. https://doi.org/10.1371/journal.pone.0023938
Loughlin P., Lin Y., Chen M. Chlorophyll d and Acaryochloris marina: current status // Photosynth. Res. 2013. V. 116. P. 277–293.
MacColl R. Cyanobacterial phycobilisomes // J. Struct. B-iol. 1998. V. 124. P. 311−334.
Manning W.M., Strain H.H. Chlorophyll d, a green pigment of red algae // J. Biol. Chem. 1943. V. 151. P. 1‒19.
Martinez-Garcia M., Koblızek M., Lopez-Legentil S., Anton J. Epibiosis of oxygenic phototrophs containing chlorophylls a, b, c and d on the colonial ascidian Cystodytes dellechiajei // Microb. Ecol. 2011. V. 61. P. 13–19.
Mascoli V., Bhatti A.F., Bersanini L., van Amerongen H., Croce R. The antenna of far-red absorbing cyanobacteria increases both absorption and quantum efficieny of Photosystem II // Nat. Commun. 2022. V. 13. Art. 3562. https://doi.org/10.1038/s41467-022-31099-5
McNamara C.J., Perry-VI T.D., Bearce K.A., Hernandez-Duque G., Mitchell R. Epilithic and endolithic bacterial communities in limestone from a Mayan archaeological site // Microb. Ecol. 2006. V. 51. P. 51–64.
Miao D., Ding W.-L., Zhao B.-Q., Lu L., Xu Q.-Z., Scheer H., Zhao K.-H. Adapting photosynthesis to the near-infrared: non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC 7335 // Biochim. Biophys. Acta. 2016. V. 1857. P. 688–694.
Miller S.R., Augustine S., Olson T.L., Blankenship R.E., Selker J., Wood A.M. Discovery of a free-living chlorophyll d-producing cyanobacterium with a hybrid proteobacterial cyanobacterial small-subunit rRNA gene // Proc. Natl. Acad. Sci. USA. 2005. V. 102. P. 850‒855.
Miller S.R., Wood A.M., Blankenship R.E., Kim M.N., Ferriera S. Dynamics of gene duplication in the genomes of chlorophyll d-producing cyanobacteria: implications for the ecological niche // Genome Biol. Evol. 2011. V. 3. P. 601–613. https://doi.org/10.1093/gbe/evr060
Mimuro M., Akimoto S., Gotoh T., Yokono M., Akiyama M., Tsuchiya T., Miyashita H., Kobayashi M., Yamazaki I. Identification of the primary electron donor in PS II of the Chl d-dominated cyanobacterium Acaryochloris marina // FEBS Lett. 2004. V. 556. P. 95–98.
Mimuro M., Akimoto S., Yamazaki I., Miyashita H., Miyachi S. Fluorescence properties of chlorophyll d-dominating prokaryotic alga, Acaryochloris marina: studies using time-resolved fluorescence spectroscopy on intact cells // Biochim. Biophys. Acta. 1999. V. 1412. P. 37–46.
Mimuro M., Hirayama K., Uezono K., Miyashita H., Miyachi S. Uphill energy transfer in a chlorophyll d-dominating oxygenic photosynthetic prokaryote, Acaryochloris marina // Biochim. Biophys. Acta. 2000. V. 1456. P. 27–34.
Miyashita H., Adachi K., Kurano N., Ikemoto H., Chihara M., Miyachi S. Pigment composition of a novel oxygenic photosynthetic prokaryote containing chlorophyll d as the major chlorophyll // Plant Cell Physiol. 1997. V. 38. P. 274–281.
Miyashita H., Ikemoto H., Kurano N., Adachi K., Chihara M., Miyachi S. Chlorophyll d as a major pigment // Nature. 1996. V. 383. P. 402.
Miyashita H., Ikemoto H., Kurano N., Miyachi S., Chihara M. Acaryochloris marina gen. et sp. nov. (Cyanobacteria), an oxygenic photosynthetic prokaryote containing chlorophyll d as a major pigment // J. Phycol. 2003. V. 39. P. 1247‒1253.
Miyashita H., Ohkubo S., Komatsu H., Sorimachi Y., Fukayama D., Fujinuma D., Akitsu S., Kobayashi M. Discovery of chlorophyll d in Acaryochloris marina and chlorophyll f in a unicellular cyanobacterium, strain KC1, isolated from Lake Biwa // J. Phys. Chem. Biophys. 2014. V. 4. Art. 149. https://doi.org/10.4172/2161-0348.1000149
Mohr R., Voß B., Schliep M., Kurz T., Maldener I., Adams D.G., Larkum A.W.D., Chen M., Hess W.R. A new chlorophyll d-containing cyanobacterium: evidence for niche adaptation in the genus Acaryochloris // ISME J. 2010. V. 4. P. 1456–1469.
Murakami A., Miyashita H., Iseki M., Adachi K., Mimuro M. Chlorophyll d in an epiphytic cyanobacterium of red algae // Science. 2004. V. 303. P. 1633.
Murray J.W. Sequence variation at the oxygen evolving centre of photosystem II: a new class of ‘rogue’ cyanobacterial D1 proteins // Photosynth. Res. 2012. V. 110. P. 177–184.
Neerken S., Amesz J. The antenna reaction center complex of heliobacteria: composition, energy conversion and electron transfer // Biochim. Biophys. Acta. 2001. V. 1507. P. 278‒290.
Niedzwiedzki D.M., Bar-Zvi S., Blankenship R.E., Adir N. Mapping the excitation energy migration pathways in phycobilisomes from the cyanobacterium Acaryochloris marina // Biochim. Biophys. Acta Bioenerg. 2019. V. 1860. P. 286‒296.
Niedzwiedzki D.M., Liu H., Chen M., Blankenship R.E. Excited state properties of chlorophyll f in organic solvents at ambient and cryogenic temperatures // Photosynth. Res. 2014. V. 121. P. 25‒34.
Nürnberg D.J., Morton J., Santabarbara S., Telfer A., Joliot P., Antonaru L., Ruban A., Cardona T., Krausz F., Bousac A., Fantuzzi A., Rutherford A.W. Photochemistry beyond the red limit in chlorophyll f-containing photosystems // Science. 2018. V. 360. P. 1210‒1213.
Ohkubo S., Miyashita H. Selective detection and phylogenetic diversity of Acaryochloris spp. that exist in association with didemnid ascidians and sponge // Microb. Environ. 2012. V. 27. P. 217–225.
Ohkubo S., Miyashita Y., Murakami A., Takeyama H., Tsuchiya T., Mimuro M. Molecular detection of epiphytic Acaryochloris spp. on marine macroalgae // Appl. Environ. Microbiol. 2006. V. 72. P. 7912–7915.
Partensky F., Six C., Ratin M., Garczarek L., Vaulot D., Probert I., Calteu A., Gourvil P., Marie D., Grébert T., Bouchier C., Le Panse S., Gachenot M., Rodriguez F., Garrido J.L. A novel species of the marine cyanobacterium Acaryochloris with a unique pigment content and lifestyle // Sci. Rep. 2018. V. 8. Art. 9142. https://doi.org/10.1038/s41598-018-27542-7
Pinevich A., Averina S. New life for old discovery: amazing story about how bacterial predation on Chlorella resolved a paradox of dark cyanobacteria and gave the key to early history of oxygenic photosynthesis and aerobic respiration // Protistology. 2021. V. 15. P. 107‒126.
Renger T., Schlodder E. The primary electron donor of Photosystem II of the cyanobacterium Acaryochloris marina is a chlorophyll d and the water oxidation is driven by a chlorophyll a/chlorophyll d heterodimer // J. Phys. Chem. 2008. V. 112. P. 7351–7354.
de los Rios A., Grube M., Sancho L.G., Ascaso C. Ultrastructural and genetic characteristics of endolithic cyanobacterial biofilms colonizing Antarctic granite rocks // FEMS Microb. Ecol. 2007. V. 59. P. 386–395.
Sawicki A., Chen M. Molecular mechanism of photosynthesis driven by red-shifted chlorophylls // Microbial Photosynthesis / Eds. Wang Q. et al. Singapore: Springer, 2020. https://doi.org/0.1007/978-981-15-3110-1_1
Schiller H., Senger H., Miyashita H., Miyachi S., Dau H. Light-harvesting in Acaryochloris marina ‒ spectroscopic characterization of a chlorophyll d-dominated photosynthetic antenna system // FEBS Lett. 1997. V. 30. P. 433‒436.
Schliep M., Chen M., Larkum A., Quinnell R. The function of MgDVP in a chlorophyll d-containing organism // Photosynthesis. Energy from the Sun / Eds. Allen J.F. et al. Dordrecht: Springer, 2008. P. 1125‒1128.
Schliep M., Crossett B., Willows R.D., Chen M. 18O labeling of chlorophyll d in Acaryochloris marina reveals that chlorophyll a and molecular oxygen are precursors // J. Biol. Chem. 2010. V. 285. P. 28450–28456.
Schmitt F.-J., Campbell Z.Y., Bui M.V., Hüls A., Tomo T., Chen M., Maksimov E.G., Allakhverdiev S.I., Friendrich T. Photosynthesis supported by a chlorophyll f-dependent, entropy-driven uphill energy transfer in Halomicronema hongdechloris cells adapted to far-red light // Photosynth. Res. 2019. V. 139. P. 185‒201.
Shen G., Canniffe D.P., Ho M.Y., Kurashov V., van der Est A., Golbeck J.Y., Bryant D.A. Characterization of chlorophyll f synthase heterologously produced in Synechococcus sp. PCC 7002 // Photosynth. Res. 2019. V. 140. P. 77–92.
Shen L.-Q., Zhang Z.-C., Shang J.-L., Li Z.-K., Chen M., Li R., Qiu B.-S. Kovacikia minuta sp. nov. (Leptolyngbyaceae, Cyanobacteria), a new freshwater chlorophyll f-producing cyanobacterium // J. Phycol. 2022. Accepted. https://doi.org/10.1111/jpy.13248
Soulier N., Laremore T.N., Bryant D.A. Characterization of cyanobacterial allophycocyanins absorbing far-red light // Photosynth. Res. 2020. V. 145. P. 189–207.
Swingley W.D., Chen M., Cheung P.C., Conrad A.L., Dejesa L.C., Hao J., Honchak B.M., Karbach L.E., Kurdoglu A., Lahiri S., Mastrian S.D., Miyashita H., Page L., Ramakrishna P., Satoh S., Sattley W.M., Shimada Y., Taylor H.L., Tomo T., Tsuchiya T., Wang Z.T., Raymond J., Mimuro M., Blankenship R.E., Touchman J.W. Niche adaptation and genome expansion in the chlorophyll d producing cyanobacterium Acaryochloris marina // Proc. Natl. Acad. Sci. USA. 2008. V. 105. P. 2005–2010.
Swingley W.D., Hohmann-Marriott M.F., Olson T.L., Blankenship R.E. Effect of iron on growth and ultrastructure of Acaryochloris marina // Appl. Environ. Microbiol. 2005. V. 71. P. 8606‒8610.
Tomo T., Kato Y., Suzuki T., Akimoto S., Okubo T., Noguchi T., Hasegawa K., Tsuchiya T., Tanaka K., Fukuya M., Dohmae N., Watanabe T., Mimuro M. Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017 // J. Biol. Chem. 2008. V. 283. P. 18198–18209.
Tomo T., Okubo T., Akimoto S., Yokono M., Miyashita H., Tsuchiya T., Noguchi T., Mimuro M. Identification of the special pair of photosystem II in a chlorophyll d dominated cyanobacterium // Proc. Natl. Acad. Sci. USA. 2007. V. 104. P. 7283–7288.
Tomo T., Shinoda T., Chen M., Allakhverdiev S.I., Akimoto S. Energy transfer processes in chlorophyll f-containing cyanobacteria using time-resolved fluorescence spectroscopy on intact cells // Biochim. Biophys. Acta. 2014. V. 1837. P. 1484–1489.
Trampe E., Kühl M. Chlorophyll f distribution and dynamics in cyanobacterial beachrock biofilms // J. Phycol. 2016. V. 52. P. 990‒996.
Trinugroho J.P., Bečková M., Shao S., Yu J., Zhao Z., Murray J.W., Sobotka R., Komenda J., Nixon P.J. Chlorophyll f synthesis by a super-rogue photosystem II complex // Nat. Plants. 2020. V. 6. P. 238–244.
Tsuzuki Y., Tsukatani Y., Yamakawa H., Itoh S., Fujita Y., Yamamoto H. Effects of light and oxygen on chlorophyll d biosynthesis in a marine cyanobacterium Acaryochloris marina // Plants. 2022. V. 11. Art. 915. https://doi.org/10.3390/plants11070915
Ulrich N.J., Uchida H., Kanesaki Y., Hirose E., Murakami A., Miller S.R. Reacquisition of light-harvesting genes in a marine cyanobacterium confers a broader solar niche // Curr. Biol. 2021. V. 31. P. 1539‒1546.
Wood A.M. Acaryochloris ‒ explaining the riddle of chlorophyll d in red algae and expanding PAR for oxygenic photosynthesis // J. Phycol. V. 48. P. 1317‒1319.
Xu C., Zhu Q., Chen J.-H., Shen L., Yi X., Huang Z., Wang W., Chen M., Kuang T., Shen J.-R., Zhang X., Han G. A unique photosystem I reaction center from a chlorophyll d-containing cyanobacterium Acaryochloris marina // J. Integr. Plant Biol. 2021. V. 63. P. 1740‒1752. https://doi.org/10.1111/jipb.13113
Yoneda A., Wittmann B.J., King J.D., Blankenship R.E., Dantas G. Transcriptomic analysis illuminates genes involved in chlorophyll synthesis after nitrogen starvation in Acaryochloris sp. CCMEE 5410 // Photosynth. Res. 2016. V. 129. P. 171–182.
Zhang Z.-C., Li Z.-K., Yin Y.-C., Li Y., Jia Y., Chen M., Qiu B.-S. Widespread occurrence and unexpected diversity of red-shifted chlorophyll-producing cyanobacteria in humid subtropical forest ecosystems // Environ. Microbiol. 2019. V. 21. P. 1497‒1510. https://doi.org/10.1111/1462-2920.14582
Zhao C., Gan F., Shen G., Bryant D.A. RfpA, RfpB, and Rf-pC are the master control elements of far-red light photoacclimation (FaRLiP) // Front. Microbiol. 2015. V. 6. Art. 1303. https://doi.org/10.3389/fmicb.2015.01303
Дополнительные материалы отсутствуют.


