Микология и фитопатология, 2019, T. 53, № 1, стр. 10-16
New species and new findings in Russia of Alternaria sect. Gypsophilae
1 Всероссийский НИИ защиты растений
Санкт-Петербург, Россия
* E-mail: fgannibal@vizr.spb.ru
Поступила в редакцию 11529.031005.201720172018
Аннотация
Alternaria sect. Gypsophilae consists of 9 species that have been isolated from plants in the Caryophyllaceae. Findings of only one species, A. nobilis, in Russia were published previously. This work was dedicated to identification of Alternaria sect. Gypsophilae-like fungi collected in Russia and to reconstruction of host shift history in this section. Alternaria fungi from Caryophyllaceae were analyzed by morphology or morphology and mole-cular phylogeny. 10 appropriate herbarium specimens were found in Herbaria LE and LEP and nine strains were taken from the State Collection of Microorganisms Pathogenic for Plants and Pests of the All-Russian Institute of Plant Protection (VIZR). Identification of four A. saponariae herbarium specimens was confirmed. Two species were revealed among pure cultures: A. saponariae and a species from Dianthus barbatus described as A. kamtschatica sp. nov. Cladogram analysis allowed assumption that host specialization in Alternaria sect. Gypsophilae shifted in the following direction: Vaccaria and Gypsophilae → Dianthus → Saponaria.
Alternaria Nees 1816 is a large, morphologically diverse genus that comprises almost 300 distinguishable morphospecies (Simmons, 2007). Recently, a number of molecular phylogenetic studies have attempted to better resolve the phylogeny of Alternaria and related alternarioid hyphomycetes (Pryor, Bigelow 2003; Hong et al., 2005; Runa et al., 2009; Lawrence et al., 2012, 2013, 2014; Woudenberg et al., 2013, 2014; Armitage et al., 2015). In total up to date, 27 sections were described in the genus.
Eleven Alternaria species were described exclusively from caryophyllaceous hosts (Simmons, 2002, 2007). Due to series of molecular phylogenetic analyses nine of those species were combined into separate section Gypsophilae D.P. Lawr., Gannibal, Peever et B.M. Pryor (Lawrence et al., 2013; Gannibal, Lawrence, 2018). Conidia of Alternaria sect. Gypsophilae are formed in short simple or branching chains, ellipsoid or globose, broadly or narrowly ovoid with the basal conidium usually obclavate. Conidia are small or large with many transverse and longitudinal septa, conspicuous constriction at some transverse septa.
Species of Alternaria sect. Gypsophilae are rather rare. They were collected in different parts of the world but only a few times each (Simmons, 2007). Probably occasional findings are consequences of sparse distribution and non-agricultural nature of most plant hosts of those fungi.
To our knowledge only one Alternaria sect. Gypsophilae species [A. nobilis (Vize) E. G. Simmons] was previously definitely revealed in Russia. It has been found on two Dianthus species and Saponaria officinalis on the south of the Far East (Osipyan, 1975; Nelen, 1972; Egorova, 1999; Egorova, Pavlyuk, 2006) and the center of European part of Russia (Sinadskiy et al., 1990) as well as in the greenhouses in the North western part of the country (Akhmed, 1990). Significantly that A. nobilis originally has not been found on Saponaria, when another similar fungus, A. saponariae, has been revealed on this plant. Therefore very likely that specimen of A. nobilis from Saponaria was misidentified and at least two species of Alternaria sect. Gypsophilae can be found in Russia.
There was also a finding on Cerastium maximum in Yakutia designated with an incorrect name Macrosporium dianthi Almeida et Camara and containing no sample description (Shkarupa, 1980). Then it was re-published and loosely synonymized as Alternaria saponariae (Karatygin et al., 1999).
Furthermore some Alternaria spp. from Caryophyllaceae belong to other sections. Alternaria dianthicola Neergaard from sect. Dianthicola was noted on Dianthus barbatus, D. arenarius, D. varyophyllus, and D. plumarius in Primorskiy krai (Nelen, 1972; Egorova, 1999; Egorova, Pavlyuk, 2006). Also some ubiquitous saprotrophic species from A. sect. Alternaria and Alternaria sect. Infectoriae can be widely recovered from almost any plants including Caryophyllaceae.
The aims of this work were to identify Alternaria sect. Gypsophilae-like herbarium specimens and strains from main Russian fungal collections and to recovery host shift history in this section.
MATERIALS AND METHODS
Two main Russian mycological herbariums and two appropriate pure culture collections were checked for presence of Alternaria sect. Gypsophilae specimens or strains, respectively. Eight and two relevant specimens were found in Mycological herbarium of the V.L. Komarov Botanical Institute (LE) and All-Russian Institute of Plant Protection (LEP), respectively. Identification of all herbarium specimens was verified by study of their morphology.
The State Collection of Microorganisms Pathogenic for Plants and Pests of the All-Russian Institute of Plant Protection (VIZR) contains nine strains of interest. Molecular phylogeny and morphology was analyzed for those isolates. No relevant Russian strains were revealed in the All-Russian Collection of Microorganisms (VKM).
For examination of the conidial morphology isolates were grown on PCA (potato carrot agar; Simmons 2007) and V4 (Mikhailova et al., 2002) that is analogue of V8 media using for description of large-spored and other Alternaria species (Simmons, 2007). Isolates were incubated for 7–14 days at 23 ± 1°С without expose under light (for cultural study) or under an alternating light/dark cycle consisting of 12 h of cool-white fluorescent daylight (for morphological study). Species identification was performed with the Alternaria identification manual (Simmons 2007).
To harvest mycelium for DNA extraction strains were grown in Petri dishes containing Czapek agar for 7–10 days. DNA was isolated using standard “СТАВ/chloroform” extraction method (Doyle, Doyle, 1987). Amplification of portions of three protein coding genes, glyceraldehyde-3-phosphate dehydrogenase (gpd), calmodulin (cald), and Alternaria major allergen (Alt a 1), utilized primer pairs gpd1/gpd2, CALDF1/CALDR1, and Alt-for/Alt-rev, respectively (Berbee et al. 1999; Lawrence et al. 2013; Hong et al. 2005, respectively). PCR products were visualized on a 1.5% agarose gel to validate presence and size of amplicons. Then amplicons were purified and sequenced on an ABI 3500 Capillary Electrophoresis Genetic Analyzer according to manufacturer’s instructions in the Core Centrum Innovative Technologies of Plant Protection (All-Russian Institute of Plant Protection, St. Petersburg, Russia).
Sequences were checked and corrected by use of ContigExpress of Vector NTI Suite 8.0 and then deposited in GenBank (https://www.ncbi.nlm.nih.gov), nos. MG050714–MG050734. ClustalX 1.8 software (Thompson et al., 1997) was used to align sequences. Phylogeny was inferred with maximum likelihood algorithm using RAxML (randomized accelerated maximum likelihood) software (v. 7.2.8, Stamatakis et al., 2006). Topology confidence was assessed with bootstrap analysis with 1000 replicates. Sixteen sequences of ex-type or representative strains of eight Alternaria sect. Gypsophilae species were downloaded from GenBank and used for phylogenetic analysis (Table 1). A. radicina was used as an outgroup species.
Table 1.
GenBank accession numbers for sequences of ex-type or representative Alternaria sect. Gypsophilae strains used for phylogenetic analysis
| Species | Strain | GenBank accession numbers | ||
|---|---|---|---|---|
| Alt a1 | cald | gpd | ||
| A. axiaeriisporifera | CBS 118715 | KC584101 | ||
| A. ellipsoidea | CBS 119674 | KC584115 | ||
| A. juxtiseptata | CBS 119673 | KC584122 | ||
| A. saponariae | CBS 116492 | KC584135 | ||
| A. gypsophilae | CBS 107.41 | JQ646387 | JQ646193 | KC584118 |
| A. nobilis | CBS 116490 | JQ646385 | JQ646191 | KC584127 |
| A. vaccariae | CBS 116533 | JQ646386 | JQ646192 | KC584146 |
| A. vaccariicola | CBS 118714 | JQ646384 | JQ646190 | KC584147 |
| A. radicina (outgroup) | ATCC 96831 | AY563286 | JQ646185 | AY278797 |
RESULTS AND DISCUSSION
Herbarium specimens. Identification of four specimens of A. saponariae (= Macrosporium saponariae Peck) was confirmed:
LE 162096 – on living leaves of Saponaria, Kurskaya guberniya, Kurskiy uezd, monastery garden, 28.07.1915; LE 162091 – on living leaves of Saponaria officinalis, St. Petersburg, Botanical Garden, 07.09.1921, leg. and det. L. Lebedeva; LEP 137154 – on leaves of Saponaria officinalis, Moskovskaya oblast, state farm Bittsa, 21.09.1936, leg. and det. N. Klaptsova; LEP 137155 – on leaves of Saponaria officinalis, Leningradskaya oblast, Lembolovo, plant nursery of LKhFI, 22.09.1969, leg. and det. V.I. Gordenko.
No typical A. saponariae conidia were found in other five specimens. They contain some small conidia similar with that of saprotrophic species of Alternaria sect. Alternaria. Recognition of A. saponariae in the following specimens labeled as “Macrosporium saponariae Peck on leaves of Saponaria officinalis” was failed: LE 162093 – Tambov, 10.07.1902, leg. Iv. Schiraevsky; LE 162098 – Tambov, 24.05.1903, leg. I. Schiraevsky; LE 162101 – surroundings of Kursk, 09.08.1915; LE 162092 – Orlovskaya guberniya, 20.08.1915, leg. V. Bondartseva, det. E. Mamontova; LE 162102 – Donetskiy okrug, Vyoshenskiy rayon (currently Rostovskaya oblast), 28.07.1928, leg. and det. M.K. Khokhryakov.
Similarly narrow A. alternata-like conidia instead of typical A. nobilis conidia were found in the specimen of Alternaria dianthi Stevens et Hall, LE 24485 – on died leaves of Dianthus deltoids, Leningradskaya oblast, Nizhnesvirskiy nature reserve, Lakhta, square 77, 01.09.1990, leg. and det. V.A. Melnik.
Molecular phylogeny of strains. Phylogenetic trees inferred from analysis of three genes and combined dataset were similar. Gpd-based tree containing the biggest number of representative isolates and combined dataset tree are presented in figures 1 and 2. In total five clusters were derived. Five VIZR isolates were in the cluster 5 together with A. saponariae. All those isolates were obtained from Saponaria oficinalis or unknown Caryophyllaceae (most likely S. oficinalis too). An isolate MF-P122-011 from Dianthus barbatus was apart of all other clusters containing any reference sequences. Nucleotide sequence difference of that strain and closely related strains was 1, 8, and 12 bp for gpd, Alt a 1 and cald, respectively.
Fig. 1.
Phylogenetic tree of Alternaria sect. Gypsophilae generated from maximum likelihood analysis of the gpd gene sequences. Numbers above nodes represents bootstrap values from 1000 replicates (only values higher than 50% are given). The scale bar indicates the number of nucleotide substitutions.
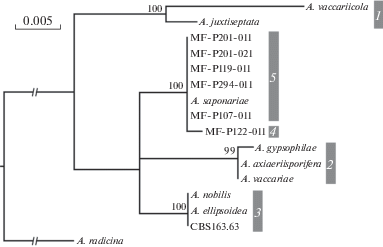
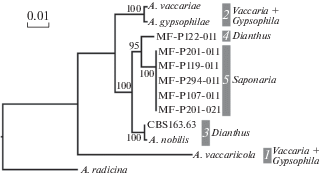
Fig. 2.
Phylogenetic tree of Alternaria sect. Gypsophilae generated from maximum likelihood analysis of gpd, cald, and Alt a1 genes combined dataset. Numbers above nodes represents bootstrap values from 1000 replicates (only values higher than 50% are given). The scale bar indicates the number of nucleotide substitutions. Host plants are listed to the right from the clades.
Morphological identification of strains. Two species were identified during analysis of VIZR pure culture collection. Six isolates from Saponaria and unidentified Caryophyllaceae collected in four places were defined as A. saponariae:
MF-P107011 – on Saponaria officinalis leaves, Lipetskaya oblast, Gryazinskiy rayon, Sukhoborie, 15.07.2005, collected by E.L. Gasich.
MF-P294011 and MF-P294021 – on S. officinalis leaves, Permskiy kray, Dobryanskiy rayon, Verkh-Kvazhva, 12.09.2009, collected by Ph.B. Gannibal.
MF-P119011 – on S. officinalis leaves, Primorskiy kray, Vladivostok, Trudovoy, 43°18.18′, 132°06.50′, 01.09.2006, collected by Ph.B. Gannibal.
MF-P201011 and MF-P201021 – on leaves of unidentified Caryophyllaceae, Irkutsk, 52°15.79′, 104°14.69′, 13.07.2008, collected by Ph.B. Gannibal.
Three isolates (MF-P122011, MF-P122021, MF-P122051) of the same origin were established as a new species and are described below.
Alternaria kamtschatica Gannibal, sp. nov. (MB 823036) (Fig. 3).
Fig. 3.
Alternaria kamtschatica strain MF-P122011: a – conidia and conidiophores from culture on PCA; b – conidia from culture on V4; c – conidia from moist chamber; d – sporulation pattern on V4. Scale bar – 50 μm.

On PCA colonies are olive; aerial mycelium is very weak or absent; diameter of 7-d old colonies is about 45–50 mm; sporulation is scanty. Primary conidiophores usually are solitary and uncrowded. They are simple or branched with 1–4 conidiogenous loci; 30–90 × 5–6 μm swollen at the apex up to 6–7 μm. Conidia form simple or branched chains of 3–5 units in a raw. Conidial “bushes” may appear in older cultures.
Juvenile conidia are pale, light brown, oval, obpyriform or subcylindric then long ellipsoid. The mature conidia are mainly obclavate. Their body is broadly or narrowly ellipsoid; usually olive brown, sometimes dark brown 35–78 × 14–22 μm. Most conidial bodies have 4–6(8) transverse septa. Longisepta present as 1–3 in some or most of transverse segments. The conidial body is slightly or deeply constricted near the transverse septa. Apical conidial cell is conical, rear subcylindrical or hemispherical. Many conidia have apical secondary conidiophores 5–19 × 3.5–4.5 μm bearing 1–3 conidiogenous loci. Sometimes basal conidia produce 1–5 lateral secondary conidiophores, 2–10 μm long.
A part of conidia remain small, narrowly obclavate almost long cylindrical. Narrow body of such conidia is gradually turning into apical secondary conidiophore bearing 1–4 conidiogenous loci. Secondary conidiophore may be longer than body. Total length of such type of conidia is 30–94 × 5.5–8 μm; secondary conidiophore can be shorter than body or two times longer or more. The conidial bodies have 1–3 transverse septa; secondary conidiophores have 0–3 transverse septa; longisepta usually are absent. Small conidia often form chains of 2–4 units, which can remain connected in preparations. Conidium size and position in a chain do not correlate.
On V4 cultures are olive and whitish due to covering sparse wooly aerial mycelium; diameter of 7-d old colonies is the same as on PCA; sporulation is good. On V4 conidia are usually negligibly shorter and wider, however solitary conidia are longer: body 35–88 × 15–25(32) μm and beak (0)10–55 × 3.5–5.0 μm. On natural substratum in a damp chamber conidia are slightly smaller than on PCA.
A. kamtschatica is morphologically very similar to another species from Dianthus, A. nobilis. The new species differs by somewhat smaller conidia size.
Holotype: LEP 014847 – dried V4 agar culture of the strain MF-P122011 (15.06.2011) isolated from leaf lesion of sweet william, Dianthus barbatus, 18.08.2010, Russia, Kamchatskiy kray, Milkovo (54°42′ N, 158°37′ E), collected by Ph. B. Gannibal.
Etymology: kamtschatica refers to the geographical name of place of finding.
Evolution. All ten species of Alternaria sect. Gypsophilae were recovered from Caryophyllaceae (four genera of tribe Caryophylleae). Nine species formed five clades on the phylogenetic tree (Figs 1, 2). Several factors (e.g. morphology, geographic origin, and host plant) can correlate with cladification. Two relatively small-spored species, A. vaccariicola and A. juxtiseptata, were combined in one clade regardless their diverse geographic and substrate origin. Other species have larger conidia and no more morphological features were detected to associate with phylogeny. Four clades contain species from different continents. Contrariwise findings from different continents were placed in different clades.
Correlation of phylogeny with the host plants was more intriguing (Fig. 2). Two clades (1, 2) simultaneously contains of species from two plants, Vaccaria and Gypsophila. Two clades (3, 4) consist of species from Dianthus and one separate species (5) was known as Saponaria-associated fungus. Cladograms assume that association with Vaccaria and Gypsophila is a basal plesiomorphic character, when association with Saponaria is an apomorphic character. Obviously, evolution of host association in Alternaria sect. Gypsophilae went in that direction: Vaccaria + Gypsophilae → Dianthus → Saponaria.
This work was supported financially by the Russian Science Foundation (RSF project N 14-26-00067).
Список литературы
Akhmed D. The most noxious fungal diseases of remontant carnation in greenhouses of Leningradskaya oblast and improvement of the disease management. Abstract Cand. Sci. thesis. Leningrad, 1990 (in Russ.).
Armitage A.D., Barbara D.J., Harrison R.J., Lane C.R., Sreenivasaprasad S., Woodhall J.W., Clarcson J.P. Discrete lineages within Alternaria alternata species group: Identification using new highly variable loci and support from morphological characters. Fungal Biol. 2015. V. 119 (11). P. 994–1006.
Berbee M.L., Pirseyedi M., Hubbard S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999. V. 91. P. 964–977.
Doyle J.J., Doyle J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987. V. 19. P. 11–15.
Egorova L.N. The genus Alternaria and allied hyphomycetes from Russian Far East. Mikologiya i fitopatologiya. 1999. V. 33 (1). P. 13–18 (in Russ.).
Egorova L.N., Pavlyuk N.A. Anamorphic fungi on ornamental plants in the Botanical Garden-Institute of Russian Academy of Sciences. Mikologiya i fitopatologiya. 2006. V. 40 (2). P. 93–100 (in Russ.).
Hong S.G., Cramer R.A., Lawrence C.B., Pryor B.M. Alt a 1 allergen homologs from Alternaria and related taxa: analysis of phylogenetic content and secondary structure. Fungal Genet. Biol. 2005. V. 42. P. 119–129.
Gannibal Ph.B., Lawrence D.P. Distribution of Alternaria species among sections. 5. Species producing conidia with many longitudinal septa. Mycotaxon. 2018. V. 133. P. 285–291.
Karatygin I.V., Nezdoiminogo E.L., Novozhilov Yu.K., Zhurbenko M.P. Russian Arctic fungi. SPb., 1999 (in Russ.).
Lawrence D.P., Gannibal Ph.B., Dugan F.M., Pryor B.M. Characterization of Alternaria isolates from the infectoria species-group and a new taxon from Arrhenatherum, Pseudoalternaria arrhenatheria sp. nov. Mycol. Progress. 2014. V. 13 (2). P. 257–276.
Lawrence D.P., Gannibal Ph.B., Peever T.L., Pryor B.M. The sections of Alternaria: formalizing species-group concepts. Mycologia. 2013. V. 105 (3). P. 530–546.
Lawrence D.P., Park M.S., Pryor B.M. Nimbya and Embellisia revisited, with nov. comb. for Alternaria celosiae and A. perpunctulata. Mycol. Progress. 2012. V. 11 (3). P. 799–815.
Mikhaylova L.A., Gogoleva S.G., Gultyaeva E.I. Interaction of strains of Bipolaris sorokiniana and wheat samples. Mikologiya i fitopatologiya. 2002. V. 36 (2). P. 63–66 (in Russ.).
Nelen E.S. Pathogenic mycoflora of ornamentals on Far East. Byulleten Glavnogo Botanicheskogo Sada. 1972. V. 83. P. 111–115 (in Russ.).
Osipyan L.L. Mikoflora of Armenian SSR. Vol. 3 Hyphomycetes. Izdatelstvo Erevanskogo Universiteta, Erevan, 1975 (in Russ.).
Pryor B.M., Bigelow D.M. Molecular characterization of Embellisia and Nimbya species and their relationship to Alternaria, Ulocladium and Stemphylium. Mycologia. 2003. V. 95. P. 1141–1154.
Runa F., Park M.S., Pryor B.M. Ulocladium systematics revisited: phylogeny and taxonomic status. Mycol. Progress. 2009. V. 8 (1). P. 35–47.
Shkarupa A.G. Flora of micromycetes of north eastern Yakutia. Novosti sistematiki nizshikh rasteniy. 1980. V. 17. P. 102–109 (in Russ.).
Simmons E.G. Alternaria themes and variations (287–304). Species on Caryophyllaceae. Mycotaxon. 2002. V. 82. P. 1–40.
Simmons E.G. Alternaria. An identification manual. Utrecht, 2007.
Sinadskiy Yu.V., Kozarzhevskaya E.F., Mukhina L.N. [et al.] Diseases and pests of introduced plats. Moscow, Nauka, 1990 (in Russ.).
Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006. V. 22. P. 2688–2690.
Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997. V. 24. P. 4876–4882.
Woudenberg J.H.C., Groenewald J.Z., Binder M., Crous P.W. Alternaria redefined. Stud. Mycol. 2013. V. 75. P. 171–212.
Woudenberg J.H.C., Truter M., Groenewald J.Z., Crous P.W. Large-spored Alternaria pathogens in section Porri disentangled. Stud. Mycol. 2014. V. 79. P. 1–47.
Ахмед Д. (Akhmed) Наиболее вредоносные грибные болезни гвоздики ремонтантной в условиях защищенного грунта Ленинградской области и совершенствование мероприятий по борьбе с ним // Автореф. дисс. … канд. биол. наук. Л., 1990. 21 с.
Егорова Л.Н. (Egorova) Род Alternaria и близкие к нему гифомицеты с Дальнего Востока России // Микология и фитопатология. 1999. Т. 33. Вып. 1. С. 13–18.
Егорова Л.Н., Павлюк Н.А. (Egorova, Pavlyuk) Анаморфные грибы на цветочных растениях в Ботаническом саду-институте ДВО РАН // Микология и фитопатология. 2006. Т. 40. Вып. 2. С. 93–100.
Каратыгин И.В., Нездойминого Э.Л., Новожилов Ю.К., Журбенко М.П. (Karatygin et al.) Грибы Российской Арктики. СПб.: Изд-во СПбГХФА, 1999. 212 с.
Михайлова Л.А., Гоголева С.Г., Гультяева Е.И. (Mikhailova et al.) Взаимодействие штаммов Bipolaris sorokiniana и образцов пшеницы // Микология и фитопатология. 2002. Т. 36. Вып. 2. С. 63–66.
Нелен Е.С. (Nelen) Патогенная микофлора цветочных растений на Дальнем Востоке // Бюллетень Главного ботанического сада. 1972. Вып. 83. С. 111–115.
Осипян Л.Л. (Osipyan) Микофлора Армянской ССР. Т. 3. Гифальные грибы. Ереван: Изд-во Ереванского ун-та, 1975. 643 с.
Синадский Ю.В., Козаржевская Э.Ф., Мухина Л.Н. [и др.] (Sinadskiy et al.) Болезни и вредители растений-интродуцентов. М.: Наука, 1990. 272 с.
Шкарупа А.Г. (Shkarupa) Флора микромицетов северо-восточной Якутии. II // Новости систематики низших растений. 1980. Т. 17. С. 102–109.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


