Микология и фитопатология, 2021, T. 55, № 1, стр. 36-50
Ферментативная и антимикробная активность полярных штаммов почвенных микроскопических грибов
Д. А. Никитин 1, *, В. С. Садыкова 2, 4, **, А. Е. Куварина 2, ***, А. Г. Дах 2, ****, М. В. Бирюков 3, *****
1 Почвенный институт им. В.В. Докучаева
119017 Москва, Россия
2 Научно-исследовательский институт по изысканию новых антибиотиков им. Г.Ф. Гаузе РАН
119021 Москва, Россия
3 Московский государственный университет имени М.В. Ломоносова
119991 Москва, Россия
4 Институт биоорганической химии им. академиков М.М. Шемякина и Ю.А. Овчинникова
117997 Москва, Россия
* E-mail: dimnik90@mail.ru
** E-mail: sadykova_09@mail.ru
*** E-mail: nastena.lysenko@mail.ru
**** E-mail: alex1_96@list.ru
***** E-mail: metrim@gmail.com
Поступила в редакцию 10.04.2020
После доработки 15.09.2020
Принята к публикации 19.11.2020
Аннотация
Оценена способность к продукции вторичных метаболитов: индуцибельных ферментов (некоторые гидролазы и оксидазы) и антибиотиков для почвенных штаммов микромицетов Арктики (Земля Франца-Иосифа, Новая Земля) и Антарктиды (оазисы Холмы Тала, Холмы Ларсеманн, Ширмахера, Земля Мери Бэрд). Максимальная эстеразная активность обнаружена у штаммов типичных антарктических видов Hyphozyma variabilis 218 и Thelebolus ellipsoideus 210 – 51 и 29 нмоль ФДА/г мицелия × час соответственно. Наибольшие значения целлюлолитической активности – 89 мкмоль глюкозы/мг биомассы – отмечены у Ascochyta pisi 192. Активности внеклеточных фенолоксидаз лакказ и пероксидаз среди протестированных штаммов не обнаружено. Антибактериальная активность к B. subtilis ATCC 6633 выявлена у 75% исследованных антарктических штаммов микромицетов. Высокоактивные штаммы выделены из богатых органикой и влагой биотопов с моховым/лишайниковым покровом. Наибольшую активность проявили Paecilomyces marquandii 166, Penicillium janczewskii 165, Penicillium roseopurpureum 169 и Thelebolus ellipsoideus 210. Антагонистическую активность по отношению к антарктическим штаммам бактерий проявляло 77% протестированных штаммов грибов. Максимальное ингибирование обнаружено у штаммов типичных для Антарктиды Antarctomyces psychrotrophicus MT303855, а также эвритопного Sarocladium kiliense MT303856. Антимикотическая активность проявилась у 42% исследованных штаммов. У 38% антарктических штаммов выявлены оба типа активности.
ВВЕДЕНИЕ
Суровые климатические условия полярных регионов обуславливают отсутствие в биогеоценозах многих групп растений и животных, но доминирование там микроорганизмов (Makhalanyane et al., 2016). Относительно полно изучено их таксономическое разнообразие и приуроченность к субстратам (Gupta et al., 2015; Maxime et al., 2017). Однако, мало информации о физиологии и биохимии штаммов Арктики и Антарктики (Cowan et al., 2014; Vaca, Chávez, 2019; Margesin, Collins, 2019). Многие штаммы экстремально холодных местообитаний, благодаря длительной географической изоляции и чрезвычайно низким температурам, имеют генетические особенности и специфику метаболизма (Lasek et al., 2017). В частности, они могут продуцировать специфические вторичные метаболиты, помогающие им выжить в экстремально холодных и сухих условиях (Hamdan, 2018; Vaca, Chávez, 2019). Вторичные метаболиты представляют собой небольшие органические молекулы, которые не важны для роста, развития и размножения, но обеспечивают им конкурентные преимущества по сравнению с другими микроорганизмами в естественной среде (Brakhage 2013; Bell et al., 2013; Vaca, Chávez, 2019). Этот класс веществ выполняет, например, такую важную экологическую функцию как химическая защита и могут выступать как факторы вирулентности (Macheleidt et al., 2016; Vaca, Chávez, 2019). Среди таких метаболитов обнаруживают антибиотики и активные при низких температурах ферменты (Gupta et al., 2015; Pudasaini et al.; 2017; Fendrihan, Negoiţă, 2017; Vaca, Chávez, 2019). Предполагается, что определенный биотехнологический потенциал имеют психрофильные и психротолерантные штаммы микромицетов, преимущественно обитающие в полярных регионах (Fendrihan, Negoiţă, 2017; Prakash et al., 2019; Singh, Kanchana, 2019; Vaca, Chávez, 2019).
Большинство работ по анализу ферментативной активности микобиоты Арктики и Антарктиды проведено в отношении экзоферментов. Особенно подробно проанализированы культуральные липазы и амилазы, активные при низких температурах, для психрофильных дрожжей (Al-Maqtari et al., 2019) и мицелиальных микромицетов (Singh, Kanchana, 2019). Так, экстремофильные штаммы видов Rhodotorula glacialis, R. psychrophenolica имеют альфа-амилазы, работающие в диапазоне от 4 до 20°С (Singh et al., 2014). Много ценных для промышленности липаз найдено у высокоширотных штаммов Alternaria sp., Aspergillus versicolor, Cladosporium cladosporioides и Phoma sp. (Prakash et al., 2019; Singh, Kanchana, 2019). Имеются данные о фосфотазной активности дрожжей экстремально холодных биотопов. Например, антарктический штамм Mrakia sp. показал высокие значения по этому параметру при температурах от 4 до 15°С (Gupta et al., 2015), а у психротолерантных изолятов Aspergillus niger и Penicillium citrinum выявлена работа фосфотаз даже при околонулевых температурах (Singh, Kanchana, 2019). В биотехнологическом плане показана перспективность хитиназ и гемицеллюлаз (манназы и ксиланазы) полярных штаммов микромицетов (Fenice et al., 2012; Vaca, Chávez, 2019).
Мало сведений в литературе содержится о таких важных для функционирования экосистем экзоферментов как целлюлазы и эстеразы у психрофильных и психротолерантных штаммов грибов (Singh, Kanchana, 2019). Так, представители родов Cadophora и Cladosporium из антарктических биотопов имеют целлюлазы, хорошо работающие при околонулевых температурах (Duarte et al., 2018). В значительной части этих работ результаты ферментативной активности оценены лишь качественно, а не количественно (Krishnan et al., 2011). Для понимания функционального потенциала микробиома биогеоценозов важно также иметь сведения о разложении сложного по строению, но распространенного полимера – лигнина, в экстремально холодных экосистемах. Сейчас нет данных, подтверждающих наличие лигнинолитической активности у штаммов микромицетов и дрожжей, выделенных из Антарктиды (Duarte et al., 2018).
Опубликовано мало статей по эндоферментам арктических и антарктических штаммов микроскопических грибов (Vaca, Chávez, 2019). В первую очередь, имеются данные по антиоксидантным энзимам – супероксиддисмутазам и каталазам (Abrashev et al., 2016; Duarte et al., 2018). Эти ферменты имеют важное значение в стрессовых условиях с чрезвычайно низкими температурами и высоким уровнем ультрафиолетого излучения (как, например, в высокогорных и полярных регионах), резко повышающим количество активных форм кислорода в клетке.
В настоящее время микологи все чаще ищут продуцентов антибиотиков в местообитаниях с экстремальным климатом, поскольку у таких штаммов обнаруживают новые и высокоэффективные биологически активные соединения (Fendrihan, Negoiţă, 2017; Singh, Kanchana, 2019). Большинство антибиотиков получено при исследовании почвенных штаммов микромицетов умеренных и тропических экосистем (Егоров, 2004; Kawaguchi et al., 2013). Возможности микромицетов с пониженным температурным оптимумом роста как продуцентов антибиотиков плохо изучены. Несмотря на это, изоляты микроорганизмов из чрезвычайно холодных экосистем являются перспективными объектами для поиска новых антибиотиков (Tosi et al., 2010). Такие исследования проведены в основном в отношении антарктических штаммов бактерий (Gesheva, 2010; Tomova et al., 2015), в то время как антимикробной активности микромицетов субстратов Антарктиды до последних лет почти не уделялось внимания (Giudice, Fani, 2016; Vaca, Chávez, 2019).
Разнообразие биологически активных соединений грибов Арктики и Антарктиды пока слабо изучено, но в последние годы появляется все больше информации об их антимикробном потенциале и способности синтезировать антибиотики. На настоящий момент большинство продуцентов полярных штаммов микромицетов относятся к роду Penicillium (Kumar et al., 2018). Например, арктический изолят P. nalgiovense синтезировал амфотерицин B (Svahn et al., 2015), а штамм Geomyces sp. 2481 – антимикотик геомицин B и антибактериальный компонент геомицин C (Vaca, Chávez, 2019). Считается, что даже известные антибиотики психрофильных и психротолерантных штаммов по структуре несколько отличаются от продуцируемых гомологов мезофильных культур грибов (Sánchez et al., 2008), что важно в борьбе с толерантными формами патогенных для человека микроорганизмами, резистентными к обычным антибиотикам.
Целью работы стала оценка потенциала ферментативной (некоторые индуцибельные гидролазы и оксидазы) и антибиотической активности ряда полярных штаммов микромицетов разных систематических групп.
МАТЕРИАЛЫ И МЕТОДЫ
Тестируемые штаммы. Для оценки антагонистической и ферментативной активности выбраны арктические и антарктические штаммы микромицетов, характерные для высокоширотных экотопов (виды родов Antarctomyces, Hyphozyma, Thelebolus); фитопатогены (представители родов Ascochyta, Botrytis, Leptosphaeria, Phoma); синтезирующие антибиотики или токсины (виды родов Botrytis, Penicillium, Sarocladium, Thelebolus); недавно описанные (не ранее 2009 г.) виды микромицетов (Atradidymella muscivora, Cadophora novi-eboraci, Exophiala tremulae, Phialocephala lagerbergii); редкие для Антарктиды виды (Eurotium niveoglaucum, Leuconeurospora polypaeciloides) (табл. 1). Из 29 протестированных изолятов микроскопических грибов 10 штаммов (роды Antarctomyces, Geomyces, Thelebolus) принадлежали к порядку Pezizales, 8 штаммов (роды Aspergillus, Penicillium, Talaromyces) – к порядку Eurotiales, 4 штамма (род Cladosporium) – к порядку Capnodiales, 2 штамма (роды Acremonium, Sarocladium) к порядку Hypocreales, 2 штамма (род Phoma) – к порядку Pleosporales, 1 штамм (род Mortierella) – к порядку Mortierellales, 1 штамм (род Cadophora) – к порядку Helotiales, 1 штамм (род Geotrichum) – к порядку Saccharomycetales. Всего протестировано 29 штаммов, относящихся к 13 родам и 23 видам.
Таблица 1.
Полярные штаммы, выбранные для оценки антагонистической и ферментативной активности
| Вид [штамм] | Характеристики местообитания | Примечание | Вид [штамм] | Характеристики местообитания | Примечание |
|---|---|---|---|---|---|
| Geomyces pannorum (Link) Sigler et J.W. Carmich. [1] | Россия, Архангельская обл., Новая Земля, Ледяная Гавань, пелозем гумусовый мерзлотный (LG-17), горизонт ССа | Э; П; С; СР | *Cladosporium cladosporioides (Fresen.) G.A. de Vries [MT303853] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, альго-бактериальный мат в Долине Ветров | Э; П; Ф |
| G. pannorum [2] | Россия, Архангельская обл., Новая Земля, мыс Желания, криозем (CJ-23), горизонт CRgCa | *Penicillium crustosum Thom [MT303854] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, почва с птичьего базара | Э; П; СР | |
| G. pannorum [3] | Архангельская обл., Новая Земля, Бухта Благополучия, криозем грубогумусовый (ВВ-10), горизонт СR | *Antarctomyces psychrotrophicus Stchigel et Guarro [MT303855] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, моховый очес ненанрушенного ландшафта | С; П; К | |
| G. pannorum [4] | Россия, Архангельская обл., Новая Земля, Ледяная Гавань, пелозем гумусовый мерзлотный (LG-12), горизонт Wca | *Sarocladium kiliense (Grütz) Summerb. [MT303856] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, моховый очес ненанрушенного ландшафта | Э; Ф; | |
| Cladosporium sp. [5] | Россия, Архангельская обл., Новая Земля, Бухта Благополучия, криозем грубогумусовый (ВВ-9), горизонт АОCa | Э; П; Ф | Aspergillus flavus Link [293] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, куртины злаков, антропогенно-нарушенный ландшафт | Э; СР; СН |
| Cladosporium sp. [6] | Россия, Архангельская обл., Новая Земля, Русская Гавань, пелозем гумусовый мерзлотный (RG-5), горизонт Wca | Э; П; Ф | A. fumigatus Fresen. [294] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, куртины злаков, антропогенно-нарушенный ландшафт | Э; СР; СН |
| Cladosporium sp. [7] | Россия, Архангельская обл., Новая Земля, Русская Гавань, пелозем мерзлотный остаточно-карбонатный (RG-7), горизонт ОСCa | Э; П; Ф | Phoma leveillei Boerema et G.J. Bollen [161] | Антарктида, ст. Молодежная, оазис Холмы Тала, озерная депрессия, глеезем с сульфидным засолением, гор-нт Oalgae-bact/W | С; П; Ф |
| Penicillium sp. [8] | Россия, Архангельская обл., Новая Земля, мыс Желания, пелозем мерзлотный остаточно-карбонатный (CJ-25), горизонт CCa | Э; П; СР | Ph. violacea (Bertel) Eveleigh [214] | Антарктида, ст. Прогресс, оазис Холмы Ларсеманн, горизонт W | С; П; Ф |
| Penicillium sp. [9] | Россия, Архангельская обл., Новая Земля, мыс Желания, пелозем мерзлотный остаточно-карбонатный (CJ-25), горизонт CCa | Э; П; СР | Sarocladium kiliense Grütz [207] | Антарктида, ст. Новолазаревская, оазис Ширмахер, нефтезагрязненный полигон | Э; П; Ф |
| Penicillium expansum Link [10] | Россия, Архангельская обл., Новая Земля, Русская Гавань, пелозем мерзлотный (RG-4), горизонт ОС | Э; П; СР | Talaromyces flavus (Klöcker) Stolk et Samson [170] | Антарктида, ст. Русская, борт долины, “каменная мостовая”, горизонт B1 | Э; СР |
| P. expansum [11] | Россия, Архангельская обл., Новая Земля, Русская Гавань, пелозем гумусовый мерзлотный (RG-5), горизонт Wca | Э; П; СР | Thelebolus ellipsoideus Brumm. et de Hoog [210] | Антарктида, ст. Прогресс, днище влажной долины с моховыми подстилками, гор-т B2fungi | С; П; К |
| Mortierella sp. [12] | Россия, Архангельская обл., Новая Земля, Ледяная Гавань, пелозем гумусовый мерзлотный (LG-12), горизонт WСа | Э; П; О | Thelebolus globosus Brumm. et de Hoog [212] | Антарктида, ст. Прогресс, оазис Холмы Ларсеманн, горизонт G1 (оглеенный) | С; П; К |
| *Cadophora luteo-olivacea (J.F.H. Beyma) T.C. Harr. et McNew [MT303851] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, примитивная почва без растительного покрова, Долина Ветров | Н; П; Ф | Th. microsporus (Berk. et Broome) Kimbr. [125] | Антарктида, ст. Молодежная, оазис Холмы Тала, влажная долина, почва под моховыми и лишайниковыми подстилками | С; П; К |
| *Acremonium zonatum (Sawada) W. Gams [MT303852] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, дернина злаковых ассоциаций | Э; Ф | Th. microsporus [202] | Антарктида, ст. Новолазаревская, оазис Ширмахер, нефтезагрязненный полигон | С; П; К |
| Geotrichum candidum Link [287] | Россия, Архангельская обл., Земля Франца-Иосифа, остров Нортбрук, куртины злаков, антропогенно-нарушенный ландшафт | Э; П; О | Th. microsporus [209] | Антарктида, ст. Прогресс, оазис Холмы Ларсеманн, днище влажной долины, “каменная мостовая” | С; П; К |
Примечание. *Культуры определены по анализу участков ITS1–ITS2 рДНК (Gluschakova et al., 2011). Буквами обозначены: Э – эвритоп; С – стенотоп полярных широт; Ф – экологически связан с растениями (часто фитопатоген); П – психротолерант/психрофил; СР – сапротроф; К – копротроф; О – олиготроф; СН – синантропный вид; Н – недавно открытый вид.
Микробиологический посев исследуемых штаммов осуществляли на ряд общепринятых агаризованных сред: Чапека (ЧА), глюкозо-пептонно-дрожжевую (ГПД), голодный агар (ГА), картофельно-декстрозный агар (КДА), сусло-агар и щелочной агар (ЩА) (рН 8–9) (Domsch, 2007). Для всех изолятов микроскопических предварительно проведена идентификация по культурально-морфологическим (Domsch, 2007) или молекулярно-биологическим признакам (по анализу участков ITS1–ITS2 рДНК). Выделение ДНК из чистых культур микромицетов проводили по методике Глушаковой с соавторами (Gluschakova et al., 2011): биомассу 5–6-суточной культуры переносили в 2 мл эппендорфы, добавляли 400 мкл стеклянных шариков (300–500 мкм диаметром) и 500 мкл лизирующего буфера (TrisBase 50 mM, NaCl 250 mM, ЭДТА 50 mM, SDS 0.3%, pH 8). Приготовленную смесь взбалтывали на вортексе на скорости 3500 об/мин в течение 15 мин, затем инкубировали 1 ч при температуре 65°С, после снова трясли на вортексе 15 мин и центрифугировали (13.4 rpm) 10 мин, отбирали надосадочную жидкость. Для амплификации региона рДНК, содержащего D1/D2 домен региона 26S рДНК, использовали праймеры ITS1f (5'CTTGGTCATTTAGAGGAAGTA) и NL4 (5'GGTCCGTGTTTCAAGACGG) и смеси для ПЦР ScreenMix (ЗАО “Евроген”, Москва). Амплификатор использовали по следующей программе: (начальная денатурация – 2 мин при температуре 96°С; затем 35 циклов: денатурация – 20 с при температуре 96°С, отжиг праймеров – 50 с при температуре 52°С, синтез ДНК – 1.5 мин при температуре 72°С; конечная достройка 7 мин при температуре 72°С). Очистку ПЦР-продукта проводили с использованием набора BigDye XTerminator Purification Kit (“Applied Biosystems”, USA). Для секвенирования использовали праймер NL4. Секвенирование ДНК проводили с помощью набора реактивов BigDye Terminator V3.1 Cycle Sequencing Kit (“Applied Biosystems”, USA) с последующим анализом продуктов реакции на секвенаторе Applied Biosystems 3130xl Genetic Analyzer в Научно-производственной компании “Синтол” (Москва). Идентификацию по полученным хроматограммам проводили, используя данные генбанка NCBI (http://blast.ncbi.nlm.nih.gov/) и СABI Bioscience Database Index Fungorum (http://www.indexfungorum.org).
Идентификацию большинства бактериальных изолятов осуществляли с помощью анализа нуклеотидных последовательностей гена 16S рРНК. ДНК экстрагировали из клеток в экспоненциальной фазе роста с помощью Трис-ЭДТА буфера (10 мМ Трис, 1 мМ ЭДТА, рН 8.0) с добавлением 5% Triton X-100. Биомассу бактерий собирали с чашек Петри, суспендировали в 500 мкл буфера, обрабатывали на вортексе в течение 5 минут при 2000 об./мин и кипятили в течение 15 минут. Затем добавляли около 100 мг стеклянных шариков (диаметром около 250–300 мкм) и обрабатывали на гомогенизаторе Minilys (Bertin instruments, США) в течение 30 с при 5000 об./мин. Далее лизаты центрифугировали в течение 3 минут при 13 400 об./мин. Супернатант использовали в качестве матрицы при постановке полимеразной цепной реакции (ПЦР). Для постановки ПЦР использовали готовую ПЦР-смесь ScreenMix (Евроген, Россия). ПЦР проводили в амплификаторе T-100 (BioRad, США). ПЦР проводили с использованием праймеров 63F + 1387R. Полученные ПЦР-продукты были очищены и секвенированы компаниями “Синтол” и “Евроген” (Москва, Россия) с использованием праймеров 1100R, 805R и 537R. Редактирование нуклеотидных последовательностей выполняли с помощью программы Chromas Lite 2.01 (http://www.technelysium.com.au). Для выравнивания, сравнения и идентификации нуклеотидных последовательностей использовали программу Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) и алгоритм BLAST базы данных GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Результаты сиквенсов культур микромицетов и бактерий размещены в генбанке NCBI (https://submit.ncbi.nlm.nih.gov/subs/).
В работе использовали штаммы микромицетов отдела Ascomycota, преимущественно порядков Eurotiales и Hypocreales. Название видов даны по телеоморфной стадии, если они формировали половые структуры при выделении и последующем культивировании на питательных средах. Для остальных аскомицетов названия приведены по анаморфной стадии. Изученные штаммы микромицетов выделены из почв Земли Франца-Иосифа, севера Новой Земли, а также антарктических оазисов Холмы Тала, Холмы Ларсеманн, Ширмахера и Земли Мери Бэрд. Штаммы хранятся в коллекции кафедры Биологии почв факультета Почвоведения МГУ им. М.В. Ломоносова. Характеристика мест отбора образцов, из которых произведено выделение исследуемых штаммов, приведена в таблице 1. Сбор образцов проведен в различных биотопах: от бесплодных и сухих “каменных мостовых”, реголитов до богатых органическим веществом и растениями, а также влажных моховых очесов и озерных сапропелей. В работе применен профильно-генетический подход почвоведения, когда образцы почвы отбирают не только из поверхностного слоя, но и из глубинных горизонтов, что важно с точки зрения генетического почвоведения (Zhelezova et al., 2019). Все почвенные образцы отобраны со всеми возможными мерами по предотвращению контаминации и до начала исследования хранились в морозильной камере при температуре –18°С. Выделение микромицетов из образцов почвы проводили методом микробиологического посева “оживленных” (выдержанных сутки при комнатной температуре) почвенных суспензий (Marfenina et al., 2016). Для минеральных почвенных горизонтов применяли сукцессионный подход при посеве, выдерживая образцы при повышенных температурах и влажности (Nikitin et al., 2017).
В качестве тест-культур для изучения антагонистической активности использованы коллекционные тест-культуры Bacillus subtilis АТСС 6633 и Aspergillus niger INA 00760, из коллекции Научно-исследовательского института по изысканию новых антибиотиков им. Г.Ф. Гаузе РАН, а также 19 грамположительных и грамотрицательных штаммов бактерий и актиномицетов, выделенных из почв Антарктиды (оазис Холмы Ларсеманн), из бактериологической коллекции кафедры Биологии почв факультета почвоведения МГУ (табл. 2).
Таблица 2.
Антарктические штаммы бактерий и актиномицетов, использованные в качестве тест-культур
| Вид, штамм | Высшие таксоны | Местообитание |
|---|---|---|
| *Bosea robiniae NR_108516.1 | P: AP: R | Антарктида, ст. Прогресс, оазис Холмы Ларсеманн, микроложбин, без мхов, минер. материал под “каменной мостовой”, горизонт В1 |
| *Brevundimonas denitrificans NR_133989.1 | P: AP: CB | |
| *Pseudomonasveronii Н NR_112075.1 | P: G: PM | |
| *Sphingomonas leidyi NR_112027.1 | P: AP: SM | |
| *S. echinoides NR_113806.1 | P: AP: SM | |
| *Sphingopyxis bauzanensis NR_117213.1 | P: AP: SM | |
| *Delftia acidivorans NR_113708.1 | P: BP: BD | Антарктида, ст. Прогресс, оазис Холмы Ларсеманн, днище влажной долины со мхами, грубый и средний песок, местами органогенный материал, горизонт О |
| *Ralstonia pickettii NR_114126.1 | P: BP: BD | |
| *Sphingomonas molluscorum NR_041399.1 | P: AP: SM | |
| *S. leidyi NR_025324.1 | P: AP: SM | |
| *Stenotrophomonas maltophiliaNR_112030.1 | P: G: X | |
| *Variovorax soli NR_043811.1 | P: BP: BD | |
| *Arthrobacter russicus NR_024783.1 | AB: AB: AM | |
| Bacillus megaterium | F: BL: BLL | |
| Bacillus sp. | F: BL: BLL | |
| Myxococcus sp. 1 | P: DP: M | |
| Myxococcus sp. 2 | P: DP: M | |
| *Streptomyces tanashiensis MT394921 | AB: AB: AM | Антарктида, ст. Молодежная, оазис Холмы Тала, Влажная долина WV-I , дно ручья, type I, профиль AD-58-43, горизонт GPalgae |
| *S. roseoviolascens MT394922 | AB: AB: AM | Антарктида, ст. Молодежная, оазис Холмы Тала, профиль AD-58-40, внешняя зона долины, структурные грунты, без макрорастительности, “безгумусная почва”, горизонт B |
Оценка ферментативной активности микромицетов. Для оценки ферментативной активности штаммов применяли метод флуориметрического определения продукта гидролиза флуоресцеина диацетата (ФДА) (Schnürer, Rosswall, 1982). Это вещество гидрофобно, поэтому вначале его растворяли в ацетоне с концентрацией 20 мг/мл, а затем 100 мкл полученного раствора разводили в 20 мл фосфатного буфера. В ячейки 96-луночного планшета вносили по 50 мкл исследуемых культуральных жидкостей (КЖ). Их получали путем выращивания культуры в колбах с неагаризованной средой и последующим фильтрованием через фильтр (диаметр пор 0.45 мкм) под вакуумом. К КЖ в планшете добавляли по 200 мкл раствора ФДА в фосфатном буфере (pH = 7.6). Планшет инкубировали при температуре 37°C в течение 1 ч. Измерение интенсивности флуоресценции проводили на микропланшетном сканере Infinite® F200 (Tecan) Ex 485 nm; Em 520 nm. По значениям интенсивности флуоресценции растворов с известной концентрацией ФДА была построена градуировочная кривая. По ней определяли концентрацию образовавшегося ФДА в исследуемых образцах.
Активность внеклеточных целлюлаз у штаммов изучали путем гидролиза суррогатного субстрата карбоксиметилцеллюлозы (КМЦ) фотометрическим определением продукта реакции (восстанавливающего сахара) после дериватизации его с применением динитросалициловой кислоты (ДНС) (Silveira et al., 2014). Реакция гидролиза осуществлялась в 100 мМ ацетат-аммонийном буфере (pH 4.5). Раствор КМЦ концентрации 20 г/л готовили путем последовательного растворения его небольшими порциями в буфере на магнитной мешалке для предотвращения образования нерастворимых коагулятов. Для приготовления ДНС-реагента в 20 мл раствора 2M NaOH порциями, при нагревании и постоянном перемешивании, вносили 1 г динитросалициловой кислоты. После ее растворения в полученный раствор также при постоянном помешивании вносили 30 г сухой сегнетовой соли. После полного растворения продолжали перемешивание до охлаждения раствора. Его объем доводили до 100 мл дистиллированной водой, раствор до использования хранился в темноте. Перед измерением активности исследуемых КЖ готовили серию стандартных растворов глюкозы в водном растворе ацетатного буфера с концентрациями: 0.05, 0.025, 0.01, 0.0075, 0.0050 мг/мл. Добавляли 450 мкл полученных растворов в эппендорфы объемом 1.5 мл, доливали в них по 50 мкм КЖ. Затем вносили по 400 мкл заранее приготовленного раствора 2% КМЦ в ацетатно-аммонийном буфере. Эппендорфы инкубировали в течение 1 ч при температуре 37°C. После этого в них добавляли по 450 мкл ДНС-реагента, а в микропробирки на 1.5 мл со стандартными растворами глюкозы – по 450 мкл ДНС-реагента. Все эппендорфы нагревали до 95°C в течение 5 мин. Полученные суспензии центрифугировали в течение 3 мин при частоте 12 000 оборотов в минуту на центрифуге Миниспин (Эппендорф). После охлаждения до комнатной температуры отбирали по 100 мкл супернатанта из каждого эппендорфа в 96-луночный планшет. Измерение оптической плотности при длине волны 540 нм анализировали на микропланшетном сканере Infinite® F200 (Tecan). По полученным значениям для растворов с заданной концентрацией глюкозы строили градуировочный график зависимости оптической плотности от концентрации глюкозы. По графику вычисляли молярную концентрацию восстанавливающего сахара, образовавшегося в ходе ферментативной реакции в исследуемых КЖ.
Измерение активности внеклеточных фенолоксидаз проводили с помощью фотометрической реакции с 2,2'-азино-бис-3-этилбензотиазолин-6-сульфоновой кислотой (АБТС) (Johannes, Majcherczyk, 2000). Реакцию осуществляли следующим образом: в лунки 96-луночного микропланшета помещали 50 мкл исследуемой КЖ, а затем во все лунки раскапывали по 100 мкл 2 мМ раствора АБТС. Субстрат для реакции готовили так: 27 мг АБТС растворяли в 25 мл дистиллированной воды. В итоге получали концентрацию 2 ммоль/л. Планшет помещался в сканер Infinite® F200 и через заданные интервалы времени проводилось измерение увеличения оптической плотности при длине волны 420 нм. Перед анализом оптической плотности растворы взбалтывались в течение секунды. Через 15 секунд после снятия показаний с прибора повторно перемешивали растворы и опять измеряли эту величину. Такие манипуляции повторяли в течение 3 минут для определения скорости изменения оптической плотности раствора. По скорости его окрашивания в лунках анализировали активность ферментов.
Активность пероксидаз проводилась аналогично предыдущему эксперименту для внеклеточных фенолоксидаз, но добавляли перекись водорода в качестве субстрата (Takagi et al., 1995).
Оценка антимикробной активности микромицетов. Штаммы с антимикробной активностью выявляли с помощью метода диффузии антибиотических веществ в агар (Egorov, 2004). Поверхность агаризованной среды в чашках Петри засевали газоном тест-культуры. На среду раскладывали блоки тестируемых штаммов микромицетов. Чашки инкубировали в термостате при 25–28°С. Через 24 ч их просматривали на наличие зон ингибирования роста тест-организма вокруг блоков. Считали культуры высокоактивными, если зона ингибирования роста микроорганизма составляла более 20 мм; умеренной активностью – диаметр зоны ингибирования в 10–20 мм, а слабоактивной – менее 10 мм.
Экстракцию антибиотических веществ из культуральной жидкости (КЖ) у высокоактивных штаммов проводили этилацетатом в соотношении КЖ/этилацетат (3/1) и бутанолом в соотношении КЖ/бутанол (5/1). Экстракты упаривали при 42°C до сухого остатка в вакууме досуха на роторном испарителе “Rotavapor‑RBüchi” (Швейцария). Сухой остаток растворяли в водном 70%‑ном этаноле. Антибиотическую активность КЖ до экстракции определяли с помощью метода лунок. Антибиотическую активность экстрактов КЖ определяли с помощью метода дисков. На стерильные бумажные диски (НИИ Пастера, Россия) наносили 100 мкг экстракта на диск и высушивали в стерильных условиях. Контролем чувствительности тест-организма служили стандартные диски с нистатином для грибов (НИИ Пастера, 80 мкг/мл) и ампициллином для бактерий (НИИ Пастера, 10 мкг/мл) (Balouiri et al., 2016). Высушенные диски с экстрактами КЖ раскладывали на засеянные тест-культурами чашки Петри с агаризованной питательной средой. Чашки инкубировали в термостате при 25–28°С. Через 24 ч измеряли диаметры зон ингибирования роста вокруг диска.
Немицелиальные грамположительные и грамотрицательные штаммы бактерий из антарктических субстратов культивировали на глюкозо-пептонно-дрожжевой среде (ГПД) с глицерином. Для выращивания актиномицетов использовали агаризованную среду Гаузе-1 (Corry et al., 2011). Тест-культуру Bacillus subtilis ATCC 6633 выращивали на МПА, Aspergillus niger INA 00760 – на агаризованной среде Чапека (ЧА). Все эксперименты по определению антагонистической активности проведены в 3–5 повторностях. Оценка достоверности различий средних значений дана по критерию Стьюдента для уровня вероятности не менее 95% с использованием пакета программ Microsoft Excel 2016 и Statistica 10.0.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Ферментативная активность полярных штаммов микромицетов. Оценена активность гидролаз у 9 антарктических штаммов микромицетов (Antarctomyces psychrotrophicus MT303855, Ascochyta pisi 192, Coniothyrium glomeratum 127, Hyphozyma variabilis 218, Penicillium roseopurpureum 169, Phoma leveillei 161, Ph. violacea 214, Thelebolus ellipsoideus 210, T. microsporus 202). Такой выбор обусловлен следующими причинами. Часть видов (Antarcticomyces psychrotrophicus, Hyphozyma variabilis, Phoma leveillei, Ph. violacea, Thelebolus ellipsoideus, Th. microsporus) типична для антарктических субстратов (Arenz, Blanchette, 2011; Marfenina et al., 2016; Nikitin et al., 2017); другие – Ascochyta pisi, Coniothyrium glomeratum, Penicillium roseopurpureum – эвритопы, которые могли быть инвазивно привнесены на материк. Кроме того, судя по литературным данным (Krishnan et al., 2011), все виды, у которых оценивалась активность ферментов в данной работе, обладают хотя бы небольшой целлюлолитической активностью, что важно для наших исследований. Виды микромицетов, протестированные только на антагонизм по отношению к бактериям или грибам (Aspergillus sclerotiorum 137, Eurotium niveoglaucum 117, Exophiala tremulae 126, Lecanicillium fungicola 121, Paecilomyces marquandii 166, Penicillium chrysogenum 162, Sarocladium kiliense 207, Talaromyces flavus 170) редко обнаруживаются микологами в субстратах Антарктиды (Rosa et al., 2019).
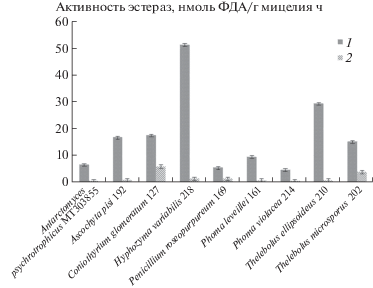
Активность наиболее распространенных гидролаз – эстераз, по которым можно оценить общую активность культур (Krishnan et al., 2011), изучена на примере разложения микромицетами флуорисцеина диацетата (ФДА). Все штаммы, росшие на среде ГПД, показали более высокие результаты активности экстераз, чем при культивации на ЧА (рис. 1). Наибольшая эстеразная активность (51 нмоль ФДА/г мицелия×час) обнаружена у штамма типичного антарктического вида Hyphozyma variabilis. У этой же культуры отмечена максимальная разница (на порядок) между значениями, полученными при культивации на двух используемых средах. ГПД богаче разнообразными субстратами для гидролаз (пептоном, водорастворимыми фракциями свободных пептидов и аминокислот, глюкозой), чем среда ЧА с единственным органическим субстратом – сахарозой. Поэтому отмечали столь существенную разницу в полученных результатах. Штамм характерного для Антарктиды Thelebolus ellipsoideus 210 на среде ГПД также проявил высокую активность эстераз (29 нмоль ФДА/г мицелия × час). Штаммы Coniothyrium glomeratum 127 и Thelebolus microsporus 202 оказались самыми активными на ЧА и показали наименьшие различия (сопоставимые с погрешностями) в выходных данных при культивировании на обоих средах. По всей видимости, эти микромицеты могут более полно усваивать относительно бедный субстрат. Особенно это актуально для психротолерантного штамма № 202, типичного для олиготрофных биотопов Антарктиды (De Hoog et al., 2004).
Рис. 1.
Активность эстераз антарктическими штаммами микромицетов, культивируемых на средах Чапека и ГПД.
Активность целлюлаз – один из важнейших параметров функционирования микроорганизмов с точки зрения экологии (Gupta et al., 2015) – исследована для 6 антарктических штаммов (Antarctomyces psychrotrophicus MT303855, Ascochyta pisi 192, Leptosphaeria coniothyrium 163, Paecilomyces marquandii 166, Phoma violacea 214, Thelebolus microsporus 202), культивируемых при различных условиях (температурах и средах). Нашей целью было сравнение способности к разложению целлюлозы штаммами в эксперименте, моделирующем микроклимат холодных (биотоп с моховым покровом; моделируемая температура 5°C) и теплых (биотоп без мохового/лишайникового покрова; моделируемая температура 25°C), богатых (биотоп – моховая долина; искусственный аналог – среда ГПД) и бедных (биотоп – пустошь; искусственный аналог – среда ЧА) органикой, полярных биотопов.
Максимальные значения целлюлолитической активности отмечены у Ascochyta pisi 192 – до 89 мкмоль глюкозы/мг сухого вещества (табл. 3). В два раза меньшие показатели – у P. violacea 214 и Antarctomyces psychrotrophicus MT303855 (до 42 мкмоль глюкозы/мг сухого вещества). Относительно высокую активность проявил штамм Thelebolus microsporus 202 – до 24 мкмоль глюкозы/мг сухого вещества. Целлюлолитическая активность других микромицетов мала (≤12 мкмоль глюкозы/мг сухого вещества). Большие результаты по этому показателю фиксировали для вариантов с используемой средой ГПД, а не с ЧА. Так, Antarctomyces psychrotrophicus MT303855, культивируемый на последней из указанных сред, практически не разлагал карбоксиметилцеллюлозу (КМЦ). Интенсивность деградации этого субстрата больше при низкой (5°C) температуре, чем при высокой (25°C) для всех тестируемых штаммов, кроме Thelebolus microsporus 202, где она оказалась примерно равной в обоих температурных условиях. Имеются данные о более полном усвоении субстрата микроорганизмами в холодном климате, по сравнению с теплым (Maggi et al., 2013). Кроме того, исследуемые изоляты выделены из антарктических биотопов, что могло снизить температурные оптимумы работы ферментов (Tosi et al., 2010; Vaz et al., 2011). Рост Th. microsporus 202 при обоих температурах очень мал. Это, очевидно, могло отразиться и на результатах целлюлолитической активности. Таким образом, также как и другим авторам (Kirtsideli et al., 2010), нам удалось показать, что для одной и той же культуры активность целлюлаз может быть высокой или низкой в зависимости от конкретных параметров эксперимента.
Таблица 3.
Активность по гидролизу (37°C) КМЦ культур антарктических штаммов микромицетов
| Вид, штамм | t, °C | Среда | Мкмоль глюкозы/1 мг сухого вещества/1 ч |
|---|---|---|---|
| Antarctomyces psychrotrophicus MT303855 | 5 | ЧА | 1 ± 1 |
| 20 | – | ||
| 5 | ГПД | 41 ± 3 | |
| 20 | 12 ± 1 | ||
| Ascochyta pisi 192 | 5 | ЧА | – |
| 20 | – | ||
| 5 | ГПД | 89 ± 10 | |
| 20 | 7 ± 1 | ||
| Leptosphaeria сoniothyrium 163 | 5 | ЧА | – |
| 20 | 8 ± 1 | ||
| 20 | ГПД | – | |
| 3 ± 1 | |||
| Paecilomyces marquandii 166 | 5 | ЧА | – |
| 20 | 3 ± 1 | ||
| 5 | ГПД | – | |
| 20 | – | ||
| Phoma violacea 214 | 5 | ЧА | – |
| 20 | – | ||
| 5 | ГПД | 42 ± 3 | |
| 20 | 2 ± 1 | ||
| Thelebolus microsporus 202 | 5 | ЧА | – |
| 20 | – | ||
| 5 | ГПД | 24 ± 2 | |
| 20 | 23 ± 2 |
Оценка активности внеклеточных фенолоксидаз лакказ и пероксидаз среди 10 антарктических штаммов (Antarctomyces psychrotrophicus MT303855, Ascochyta pisi 192, Coniothyrium glomeratum 127, Hyphozyma variabilis 218, Penicillium roseopurpureum 169, Phoma leveillei 161, Phoma violacea 214, Talaromyces flavus 170, Thelebolus ellipsoideus 210, Thelebolus microspores 202) показала, что ни у одной из проверенных культур этих ферментов не обнаружено. В Антарктиде практически полностью отсутствуют древесные растения. Видимо, поэтому большинство аборигенных штаммов не обладают активным ферментным комплексом для разложения лигнина (Kirtsideki et al., 2010) и проявляют низкую целлюлолитическую активность.
Антимикробная активность полярных штаммов микромицетов. Степень ингибирования роста бактерий у микромицетов разной таксономической принадлежности существенно отличалась. Антибактериальная активность к Bacillus subtilis ATCC 6633 выявлена у 75% протестированных антарктических штаммов микромицетов. Высокая активность (диаметр зоны ингибирования d > 20 мм) обнаружена только у 18% штаммов – Penicillium janczewskii 165, P. roseopurpureum 169, Paecilomyces marquandii 166 и Thelebolus ellipsoideus 210. Данных об антагонистических веществах, продуцируемых штаммами вида P. roseopurpureum, немного. Для этого вида сравнительно недавно описаны розеопурпурины А–H из класса поликетидов, обладающие активностью в отношении грамположительных бактерий (Shang et al., 2016). Для вида Thelebolus ellipsoideus нами впервые отмечена антимикробная активность.
Только 38% микромицетов проявляли умеренную активность (d = 15–20 мм): Ascochyta pisi 192, Botrytis cinerea 174, Cladosporium tenuissimum 184, Clonostachys rosea 138, Epicoccum nigrum 177, Phoma leveillei 161, Lecanicillium fungicola 221, Microascus cinereus 181, Microsphaeropsis olivacea 180, Penicillium simplicissimum 130, Phialocephala lagerbergii 190, Phoma leveillei 129, Ph. violacea 214 и Thelebolus microsporus 202. Среди них были штаммы типичных для Антарктиды видов (Rosa et al., 2019) – Phoma leveillei, Ph. violacea и Thelebolus microsporus.
Согласно литературным данным, для штаммов этих видов уже описан ряд антибиотиков, обладающих антибактериальным действием. Так, у штаммов вида Epicoccum nigrum в качестве антимикробных соединений с антибактериальной активностью описаны флавипин (Fávaro et al., 2012), эпиколактон и эпикокколиды А и В, ингибирующие рост Staphylococcus aureus были выделены из почвенного изолята этого вида (Nisa et al., 2015). Баверицин, полученный из эндофитного штамма, изолированного из Entada abyssinica показал высокую активность Bacillus cereus и Salmonella typhimurium с соответствующими значениями MIC 3.12 и 6.25 мкг/мл (Dzoyem et al., 2017). Вероятно, поиск продуцентов антибактериальных веществ действительно стоит проводить в первую очередь среди изолятов этих видов, в том числе выделенных из полярных регионов (Gonçalves et el., 2015).
Не проявили антибиотической активности 44% протестированных штаммов редких для Антарктиды видов (Arenz, Blanchette, 2011; Marfenina et al., 2016; Rosa et al., 2019): Arthrinium sphaerospermum 136, Eurotium niveoglaucum 117, Periconia igniaria 134, Talaromyces flavus 170. Они росли только при 25°С, поэтому полагаем, что эти штаммы не развиваются в нативных субстратах Антарктиды.
Максимальная доля активных штаммов (58%) выделена из оазисов Холмы Ларсеманн и Холмы Тала – примерно по 29% для каждого. Эти районы отбора образцов характеризуются наиболее суровыми климатическими условиями (Mergelov et al., 2016), что могло сыграть роль в продуцировании антибиотических веществ штаммами (Vaz et al., 2011; Gonçalves et el., 2015).
Характерно, что наиболее активные в подавлении Bacillus subtilis ATCC 6633 штаммы микромицетов выделены из богатых органикой и влагой биотопов моховых и лишайниковых долин, в то время как минимальной активностью обладали штаммы из бесплодных “каменных мостовых”, сульфидных солончаков и озерных грунтов. Такая закономерность выявлена и в других работах (Vaz et al., 2011; Giudice, Fani, 2016).
Антифунгальное действие в отношении Aspergillus niger INA 0076 выявлено для 42% исследуемых антарктических штаммов микромицетов. Высокой антимикотической активностью (d = 27 мм) обладал только Botrytis cinerea 174, выделенный из биотопа под моховым покровом. Умеренную активность (d = 15–20 мм) проявили 8 протестированных штаммов: Ascochyta pisi 192, Clonostachys rosea 138, Epicoccum nigrum 177, Microascus cinereus 181, Microsphaeropsis olivacea 180, Phialocephala lagerbergii 190, Phoma violacea 214, Talaromyces flavus 170. Лишь Paecilomyces marquandii 166 слабо (d = 10–15 мм) ингибировал Aspergillus niger INA 0076.
Неактивных культур (в том числе характерных для Антарктиды Phoma leveillei 129 и Thelebolus ellipsoideus 210) значительно больше – 58%. Таким образом, показано, что антарктические штаммы разных видов микромицетов проявляют, преимущественно, антибактериальную, а не антигрибную активность. Низкой антагонистической активности (d = 1–9 мм) не выявлено ни у одного из тестируемых изолятов.
Высокая антифунгальная активность Botrytis cinerea – характерное явление для представителей этого рода (Castro et al., 2019). Он редок в различных субстратах Антарктиды и, по-видимому, является заносным на материке (Rosa et al., 2019). Умеренная антифунгальная активность проявлена видами, отмечаемыми исследователями в Антарктиде (Arenz, Blanchette, 2011; Marfenina et al., 2016; Rosa et al., 2019). Часто встречающиеся в антарктических почвах Phoma leveillei и Thelebolus ellipsoideus не подавляли рост Aspergillus niger INA 00760.
Наибольшая доля активных в отношении A. niger INA 00760 штаммов (26%) выявлена из почв антарктического оазиса Холмы Ларсеманн. Единственный штамм с высокой антибактериальной активностью к Bacillus subtilis ATCC 6633. Penicillium roseopurpureum 169 выделен из субстратов оазиса Холмы Тала, где среднегодовые температуры одни из самых низких среди антарктических береговых территорий (Mergelov et al., 2016).
Более трети протестированных антарктических штаммов микромицетов (38%) ингибировали рост как Bacillus subtilis ATCC 6633, так и Aspergillus niger INA 00760. Эти микромицеты, преимущественно, имели умеренную противобактериальную и антифунгальную активности. Все представители данной группы являются эвритопами. Экстремальные абиотические факторы природной среды обитания могут стимулировать антагонизм (Gesheva, 2010), поэтому полярные экосистемы весьма интересны для исследователей, занимающихся поиском продуцентов новых и эффективных антибиотиков (Gonçalves et al., 2015).
Четыре штамма микромицетов, выделенных нами из Антарктиды (Arthrinium sphaerospermum 136, Eurotium niveoglaucum 117, Exophiala tremulae 126 и Periconia igniaria 134), не ингибировали рост ни Bacillus subtilis ATCC 6633, ни Aspergillus niger INA 00760. Эти микромицеты, несмотря на многочисленные пересевы, выделялись исключительно в виде стерильных мицелиев, не формировали органов спороношения и не продуцировали антибиотических веществ. Такие факты согласуются с известным наблюдением, что синтезу антибиотиков у грибов, как правило, предшествует стадия интенсивного конидиогенеза (Kumar, Kumar, 2019). Так, аспорогенный мутантный штамм Тrichoderma harzianum переставал синтезировать комплекс пептаиболов в сравнении с исходным “диким” изолятом с нормальным спороношением (Kubicek et al., 2007).
У 13 штаммов оценена антагонистическая активность в отношении штаммов бактерий, выделенных из почв Антарктиды (рис. 2). Выбранные штаммы грибов отобраны по следующему принципу: характерные для Антарктиды виды (Rosa et al., 2019) – Antarctomyces psychrotrophicus MT303855, Exophiala tremulae 126, Phoma leveillei 161; редкие для материка виды – Eurotium niveoglaucum 117, Talaromyces flavus 170; известные как фитопатогены – Ascochyta pisi 192, Coniothyrium glomeratum 127; известные как продуценты антибиотиков – Aspergillus sclerotiorum 137, Lecanicillium fungicola 121, Paecilomyces marquandii 166, Penicillium chrysogenum 162, Penicillium roseopurpureum 169, Sarocladium kiliense 207. Большинство (10 из 13, или 77%) протестированных изолятов проявили активность по отношению к бактериям из почв Антарктиды. Выявлено ингибирование роста только грамположительных бактерий и актиномицетов. Штаммы Eurotium niveoglaucum 117, Paecilomycesm marquandii 166, Talaromyces flavus 170 не подавляли рост ни одного из антарктических штаммов бактерий. Интересно отметить, что тот же штамм Paecilomyces marquandii 166 подавлял развитие Bacillus subtilis ATCC 6633.
Рис. 2.
Антагонистическая активность штаммов микромицетов по отношению к штаммам грамположительных бактерий, выявленная методом агаровых блоков.
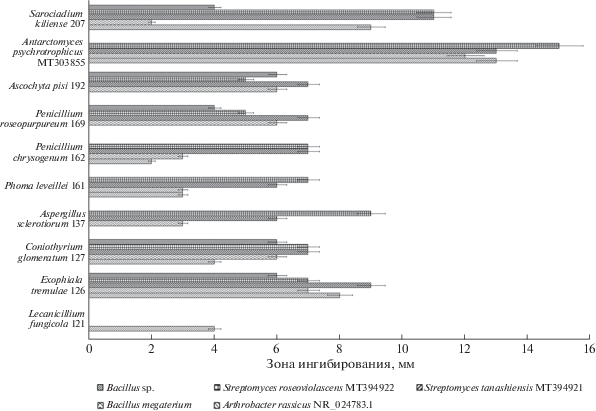
Все микромицеты проявили низкую или умеренную антагонистическую активность в отношении данных тест-культур. Максимальный антагонизм выявлен у штамма эвритопного вида Sarocladium kiliense 207 и доминантного вида многих субстратов Антарктиды – Antarctomyces psychrotrophicus MT303855. Эти штаммы выделены из биотопа богатого органикой с полноценным моховым покровом. Ранее проведено только одно исследование антибиотической активности для A. psychrotrophicus, но по отношению лишь к Escherichia coli (Abneuf et al., 2016). Активность большинства прочих штаммов оказалась невысокой. Штаммы редких для Антарктиды видов (Rosa et al., 2019) Eurotium niveoglaucum 117, Paecilomyces marquandii 166, Talaromyces flavus 170 были неактивны.
Штаммы микромицетов в большей степени подавляли рост актиномицетов, чем немицелиальных грамположительных бактерий. Так, S. kiliense 207 имел слабую антибиотическую активность к Arthrobacter russicus (NR_024783.1) и Bacillus megaterium, но умеренный диаметр зоны ингибирования для Streptomyces tanashiensis MT394921 и Streptomyces roseoviolascens MT394922. Известно, что доля актинобактерий (в том числе и актиномицетов) в субстратах Антарктиды мала (Pearce et al., 2012), это, возможно, связано с их ингибированием грибами.
Некоторые тестируемые микромицеты не подавляли рост отдельных штаммов грамположительных бактерий, выделенных из субстратов Антарктиды. Например, Antarctomyces psychrotrophicus MT303855 и Penicillium chrysogenum 162 не останавливали развитие Bacillus sp.; Ascochyta pisi 192, A. sclerotiorum 137, Lecanicillium fungicola 121 – Arthrobacter russicus NR_024783.1 и Bacillus sp.; а Penicillium roseopurpureum 169 – Arthrobacter russicus NR_024783.1.
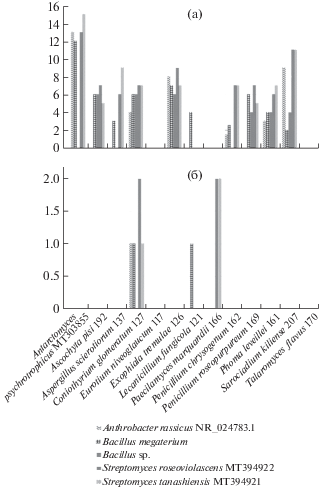
Для 13 штаммов, показавших высокую активность по методу агаровых блоков в отношении антарктических бактерий (Antarctomyces psychrotrophicus MT303855, Ascochyta pisi 192, Aspergillus sclerotiorum 137, Coniothyrium glomeratum 127, Eurotium niveoglaucum 117, Exophiala tremulae 126, Lecanicillium fungicola 121, Paecilomyces marquandii 166, Penicillium chrysogenum 162, P. roseopurpureum 169, Phoma leveillei 161, Sarocladium kiliense 207, Talaromyces flavus 170), проведена проверка активности их КЖ при росте на жидкой среде и их органических экстрактов (рис. 3). Экстракты КЖ Exophiala tremulae 126 и Lecanicillium fungicola 121 не подавляли развитие грамположительных бактерий и актиномицетов. Органические экстракты остальных штаммов подавляли рост исключительно грамположительных бактерий, в том числе актинобактерий. Только экстракт Eurotium niveoglaucum 117 слабо ингибировал штамм грамотрицательной бактерии Brevundimonas denitrificans NR_133989.1.
Рис. 3.
Антимикробная активность исследованных штаммов грибов против антарктических штаммов бактерий: А – выявленная методом агаровых блоков; Б – активность органических экстрактов КЖ.
Наибольший интерес в качестве потенциальных продуцентов антибиотических соединений вызывают типичные виды для почв Антарктиды. Среди них мы выбрали штаммы с номерами MT303855 и 218 видов Antarctomyces psychrotrophicus и Hyphozyma variabilis, соответственно.
Способность к синтезу антибиотиков у штаммов оценивали в дальнейшем на двух жидких средах: синтетической ЧА и полусинтетической, богатой ГПД. Штаммы проявили антибактериальную активность в отношении Bacillus subtilis ATCC 6633, однако не подавляли рост A. niger INA 00760. Для Antarctomyces psychrotrophicus MT303855 синтез антибиотиков был выше на среде ГПД, а для штамма 218 Hyphozyma variabilis – на минеральной среде ЧА. При этом антибиотический комплекс не извлекался органическими растворителями, а активность сохранялась в постэкстракционном остатке. Очевидно, Antarctomyces psychrotrophicus MT303855 и Hyphozyma variabilis 218 продуцируют, преимущественно, водорастворимые активные соединения. Эти типичные для полярных биотопов виды микромицетов обычно выделяют из аквальных или покрытых мхами биотопов Антарктиды (De Hoog et al., 2004; Gonçalves et al., 2012). В этих экосистемах из-за обилия водной фазы и легкодоступных органических веществ могут доминировать прокариоты, поэтому антарктическим стенотопным микромицетам приходится ингибировать их рост антибиотиками. Исследователи часто отмечают обнаружение штаммов микромицетов активных продуцентов антибиотиков в аквальных биотопах (Kümmerer, 2009). Возможно, гидрофильные антибиотические вещества эффективнее в водной среде, чем органорастворимые за счет способности с ней смешиваться.
ЗАКЛЮЧЕНИЕ
Таким образом, выделенные полярные штаммы микромицетов синтезируют биологически активные соединения, которые могут использоваться ими в экстремальных климатических условиях при конкуренции за выживание с доминирующими таксонами бактерий (Bell et al., 2013). Эти биоактивные микромицеты могут представлять собой потенциальный источник молекул-прототипов лекарственных средств (Godinho et al., 2015). В данной работе установлено, что большинство полярных штаммов микромицетов проявляют антибактериальную активность (в том числе по отношению к антарктическим штаммам бактерий), в то время как противогрибковая активность встречается гораздо реже и менее выражена. Кроме того, впервые показана антибактериальная активность типичных для Антарктиды видов – Antarctomyces psychrotrophicus и Hyphozyma variabilis, – ранее не заявленных как продуцентов антибиотиков.
Для большинства исследованных полярных штаммов свойственна сниженная функциональная активность по сравнению с изолятами из умеренного климата, что проявляется в невысоких уровнях антагонизма по отношению к другим микроорганизмам, низких уровнях целлюлазной и эстеразной активности, отсутствии фенолосидазной лакказной и пероксидазной активности. Высокая антагонистическая и ферментативная активность среди полярных штаммов редка, может выявляться только у некоторых типичных для материка видов (например, Hyphozyma variabilis, Thelebolus ellipsoideus). По-видимому, для штаммов, выделенных из почв Арктики и Антарктиды, успешная конкуренция с другими микроорганизмами не имеет определяющего значения, им гораздо важнее собственный адаптационный потенциал. Такие культуры имели максимальный рост и способность к образованию половых структур (аском) и существенное повышение ферментативной целлюлазной активности именно при низкой (5°С) температуре.
Авторы выражают благодарность м.н.с. ФГБНУ НИИНА А.А. Барановой за помощь в получении органических экстрактов для исследований антимикробной активности микромицетов. Авторы особенно признательны в.н.с. и профессору МГУ имени М.В. Ломоносова д.б.н. О.Е. Марфениной за ценные комментарии и замечания по статье. Выделение чистых культур, а также идентификация по культурально-морфологическим и молекулярно-биологическим признакам выполнено при финансовой поддержке РФФИ в рамках научного проекта № 20-04-00328. Оценка антимикробных свойств полярных штаммов микромицетов выполнена при финансовой поддержке РНФ в рамках научного проекта № 18-74-10073.
Список литературы
Abrashev R., Feller G., Kostadinova N. et al. Production, purification, and characterization of a novel cold-active superoxide dismutase from the Antarctic strain Aspergillus glaucus 363. Fungal biology. 2016. V. 120 (5). P. 679–689. https://doi.org/10.1016/j.funbio.2016.03.002
Al-Maqtari Q.A., Waleed A.-A., Mahdi A.A. Cold-active enzymes and their applications in industrial fields – A review. Int. J. Res. Agric. Sci. 2019. V. 6 (4). P. 2348–3997.
Arenz B.E., Blanchette R.A. Distribution and abundance of soil fungi in Antarctica at sites on the Peninsula, Ross Sea Region and McMurdo Dry Valleys. Soil Biology and Biochemistry. 2011. V. 43 (2). P. 308–315. https://doi.org/10.1016/j.soilbio.2010.10.016
Bell T.H., Callender K.L., Whyte L.G. et al. Microbial competition in polar soils: a review of an understudied but potentially important control on productivity. Biology. 2013. V. 2 (2). P. 533–554. https://doi.org/10.3390/biology2020533
Bhange K., Chaturvedi V., Bhatt R. Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnology reports. 2016. V. 10. P. 94–104. https://doi.org/10.1016/j.btre.2016.03.007
Brakhage A.A. Regulation of fungal secondary metabolism. Nature Reviews Microbiology. 2013. V. 11 (1). P. 21–32. https://doi.org/10.1038/nrmicro2916
Bratchkova A., Ivanova V. Bioactive metabolites produced by microorganisms collected in Antarctica and the Arctic. Biotechnology and Biotechnological Equipment. 2011. V. 25 (1). P. 1–7. https://doi.org/10.5504/BBEQ.2011.0116
Castro P., Mendoza L., Vásquez C. et al. Antifungal Activity against Botrytis cinerea of 2,6-Dimethoxy-4-(phenylimino) cyclohexa-2, 5-dienone Derivatives. Molecules. 2019. V. 24 (4). P. 706. https://doi.org/10.3390/molecules24040706
Corry J.E., Curtis G.D., Baird R.M. Handbook of culture media for food and water microbiology. Royal Society of Chemistry, 2011.
Cowan D.A., Makhalanyane T.P., Dennis P.G. et al. Microbial ecology and biogeochemistry of continental Antarctic soils. Frontiers in microbiology. 2014. V. 5. P. 154. https://doi.org/10.3389/fmicb.2014.0015
De Hoog G.S., Gottlich E., Platas G., Genilloud O. et al. Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud. Mycol. 2005. V. 51. P. 33–76.
Domsch K.H., Gams W., Anderson T.H. Compendium of soil fungi, 2nd taxonomically revised edition by W. Gams. IHW, Eching, 2007.
Dzoyem et al. Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica. Rev. Bras. Farmacogn. 2017. V. 27 (2). P. 251–253. https://doi.org/10.1016/j.bjp.2016.08.011
Egorov N.S. Antibiotics: A Scientific Approach. Moscow, 2004 (in Russ.).
Fenice M., Barghini P., Selbmann L. et al. Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003. Microbial cell factories. 2012. V. 11 (1). P. 12. https://doi.org/10.1186/1475-2859-11-12
Gesheva V. Production of antibiotics and enzymes by soil microorganisms from the windmill islands region, Wilkes Land, East Antarctica. Polar Biol. 2010. V. 33 (10). P. 1351–1357. https://doi.org/10.1007/s00300-010-0824-x
Giudice A.L., Fani R. Antimicrobial Potential of Cold-Adapted Bacteria and Fungi from Polar Regions. In Biotechnology of Extremophiles: Springer International Publishing, 2016. https://doi.org/10.1007/978-3-319-13521-2_3
Glushakova A.M., Kachalkin A.V., Chernov I.Y. Specific features of the dynamics of epiphytic and soil yeast communities in the thickets of Indian balsam on mucky gley soil. Eurasian Soil Science. 2011. V. 44 (8). P. 886–892. https://doi.org/10.1134/S1064229311080059
Gonçalves V.N., Carvalho C.R., Johann S. et al. Antibacterial, antifungal and antiprotozoal activities of fungal communities present in different substrates from Antarctica. Polar Biology. 2015. V. 38 (8). P. 1143–1152. https://doi.org/10.1007/s00300-015-1672-5
Gupta P., Sangwan N., Lal R. et al. Bacterial diversity of Drass, cold desert in Western Himalaya, and its comparison with Antarctic and Arctic. Arch. Microbiol. 2015. V. 197 (6). P. 851–860. https://doi.org/10.1007/s00203-015-1121-4
Gupta R., Kumari A., Syal P. et al. Molecular and functional diversity of yeast and fungal lipases: their role in biotechnology and cellular physiology. Progress in Lipid Research. 2015. V. 57. P. 40–54. https://doi.org/10.1016/j.plipres.2014.12.001
Hamdan A. Psychrophiles: Ecological significance and potential industrial application. South African Journal of Science. 2018. V. 114 (5–6). P. 1–6. https://doi.org/10.17159/sajs.2018/20170254
Henríquez M., Vergara K., Norambuena J. et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J. Microbiol. Biotechnol. 2014. V. 30 (1). P. 65–76. https://doi.org/10.1007/s11274-013-1418-x
Johannes C., Majcherczyk A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000. V. 78 (2). P. 193–199. https://doi.org/10.1016/S0168-1656(00)00208-X
Kawaguchi M., Nonaka K., Masuma R. et al. New method for isolating antibiotic-producing fungi. Journal of Antibiotics. 2013. V. 66 (1). P. 17–21. https://doi.org/10.1038/ja.2012.79
Kirtsideli I.Yu., Vlasov D.Yu., Abakumov E.V. et al. The diversity and enzymatic activity of micromycetes from underdeveloped soils of the Coastal Antarctic. Mikologiya i fitopatologiya. 2010. V. 44 (5). P. 387–397 (in Russ.).
Krishnan A., Alias S.A., Wong C.M. et al. Extracellular hydrolase enzyme production by soil fungi from King George Island, Antarctica. Polar biology. 2011. V. 34 (10). P. 1535–1542. https://doi.org/10.1007/s00300-011-1012-3
Kumar A., Kumar A. Synthesis and regulation of fungal secondary metabolites. Springer, Singapore, 2019. https://doi.org/10.1007/978-981-13-8844-6_2
Kümmerer K. Antibiotics in the aquatic environment – a review – part I. Chemosphere. 2009. V. 75. P. 417–434. https://doi.org/10.1016/j.chemosphere.2008.11.086
Lasek R., Dziewit L., Ciok A. et al. Genome content, metabolic pathways and biotechnological potential of the psychrophilic Arctic bacterium Psychrobacter sp. DAB_AL43B, a source and a host of novel Psychrobacter-specific vectors. J. Biotechnol. 2017. V. 263. P. 64–74. https://doi.org/10.1016/j.jbiotec.2017.09.011
Machavariani N.G., Terekhova L.P. Biologically active compounds formed by endophyte microorganisms. Antibiotics and chemotherapy. 2014. V. 59 (5-6). P. 26–33 (in Russ.).
Maggi O., Tosi S., Angelova M., Lagostina E. et al. Adaptation of fungi, including yeasts, to cold environments. Plant Biosystems. 2013. V. 147 (1). P. 247–258. https://doi.org/10.1080/11263504.2012.753135
Marfenina O.E., Nikitin D.A., Ivanova A.E. The structure of mushroom biomass and the diversity of cultivated micromycetes in the soils of Antarctica (Progress and Russkaya stations). Soil Science. 2016. V. 8. P. 991–999. (in Russ.). https://doi.org/10.7868/S0032180X16080074
Margesin R., Collins T. Microbial ecology of the cryosphere (glacial and permafrost habitats): current knowledge. Applied microbiology and biotechnology. 2019. V. 103 (4). P. 2537–2549. https://doi.org/10.1007/s00253-019-09631-3
Maxime S., Tytgat B., Verleyen E. et al. Biogeography and macroevolution in the Arctic and Antarctic lacustrine microbiomes. Abstracts. 2017.
Mergelov N.S., Dolgikh A.V., Zazovskaya E.P. et al. Soils and soil-like bodies of oases and nunataks of East Antarctica. Geography Issues. 2016. V. 142. P. 593–628 (in Russ.).
Nikitin D.A., Marfenina O.E., Maksimova I.A. Using the succession approach in studying the species composition of microscopic fungi and the content of mushroom biomass in Antarctic soils. Mikologiya i fitopatologiya. 2017. T. 51 (4). P. 211–219.
Nisa H., Kamili A.N., Nawchoo I.A. et al. Fungal endophytes as prolific source of phytochemicals and other bioactive naturalproducts: a review. Microbial Pathogenesis. 2015. V. 82. P. 50–59. https://doi.org/10.1016/j.micpath.2015.04.001
Pearce D.A., Newsham K.K., Thorne M.A. et al. Metagenomic analysis of a southern maritime Antarctic soil. Frontiers in Microbiology. 2012. V. 3. P. 403. https://doi.org/10.3389/fmicb.2012.00403
Prakash O., Mahabare K., Yadav K.K. et al. Fungi from Extreme Environments: A Potential Source of Laccases Group of Extremozymes. In: Fungi in Extreme Environments: Ecological Role and Biotechnological Significance. Springer, 2019.
Pudasaini S., Wilson J., Ji M. et al. Microbial Diversity of Browning Peninsula, Eastern Antarctica Revealed Using Molecular and Cultivation Methods. Frontiers in microbiology. 2017. V. 8. P. 591. https://doi.org/10.3389/fmicb.2017.00591
Rosa L.H., Zani C.L., Cantrell C.L. et al. Fungi in Antarctica: diversity, ecology, effects of climate change, and bioprospection for bioactive compounds. In Fungi of Antarctica. Springer, 2019. https://doi.org/10.1007/978-3-030-18367-7_1
Sánchez L.A., Gómez F.F., Delgado O.D. Cold-adapted microorganisms as a source of new antimicrobials. Extremophiles. 2008. V. 13. P. 111–120. https://doi.org/10.1007/s00792-008-0203-5
Schnürer J., Rosswall T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Applied and environmental microbiology. 1982. V. 43 (6). P. 1256–1261.
Silveira M.H.L., Aguiar R.S., Siika-aho M. et al. Assessment of the enzymatic hydrolysis profile of cellulosic substrates based on reducing sugar release. Bioresource Technology. 2014. V. 151. P. 392–396. https://doi.org/10.1016/j.biortech.2013.09.135
Singh J., Dubey A.K., Singh R.P. Antarctic terrestrial ecosystem and role of pigments in enhanced UV-B radiations. Reviews in Environmental Science and BioTechnology. 2011. V. 10 (1). P. 63–77. https://doi.org/10.1007/s11157-010-9226-3
Svahn K.S., Chryssanthou E., Olsen B. et al. Penicillium nalgiovense Laxa isolated from Antarctica is a new source of the antifungal metabolite amphotericin B. Fungal Biology and Biotechnology. 2015. V. 2 (1). P. 1. https://doi.org/10.1186/s40694-014-0011-x
Tomova I., Stoilova-Disheva M., Lazarkevich I. et al. Antimicrobial activity and resistance to heavy metals and antibiotics of heterotrophic bacteria isolated from sediment and soil samples collected from two Antarctic islands. Frontiers in Life Science. 2015. V. 8 (4). P. 348–357. https://doi.org/10.1080/21553769.2015.1044130
Tosi S., Kostadinova N., Krumova E. et al. Antioxidant enzyme activity of filamentous fungi isolated from Livingston Island, Maritime Antarctica. Polar biology. 2010. V. 33 (9). P. 1227–1237. https://doi.org/10.1007/s00300-010-0812-1
Vaca I., Chávez R. Bioactive compounds produced by Antarctic filamentous fungi. In Fungi of Antarctica. Springer, 2019.
Vaz A.B., Rosa L.H., Vieira M.L. et al. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Brazilian J. Microbiol. 2011. V. 42 (3). P. 937–947. https://doi.org/10.1590/S1517-83822011000300012
Zhelezova A., Chernov T., Tkhakakhova A. et al. Prokaryotic community shifts during soil formation on sands in the tundra zone. PloS one. 2019. V. 14 (4). https://doi:. pone.0206777 Егоров Н.С. (Egorov) Основы учения об антибиотиках. М.: МГУ, 2004. С. 528.https://doi.org/10.1371/journal
Кирцидели И.Ю., Власов Д.Ю., Абакумов Е.В. и др. (Kirtsideli et al.) Разнообразие и ферментативная активность микромицетов из слаборазвитых почв Береговой Антарктики // Микология и фитопатология. 2010. Т. 44. № 5. С. 387–397.
Марфенина О.Е., Никитин Д.А., Иванова А.Е. (Marfenina et al.) Структура грибной биомассы и разнообразие культивируемых микромицетов в почвах Антарктиды (станции Прогресс и Русская) // Почвоведение. 2016. Т. 8. С. 991–999. https://doi.org/10.7868/S0032180X16080074
Мачавариани Н.Г., Терехова Л.П. (Machavariani et al.) Биологически активные соединения, образуемые микроорганизмами-эндофитами // Антибиотики и химиотерапия. 2014. Т. 59. № 5–6. С. 5–6.
Мергелов Н.С., Долгих А.В., Зазовская Э.П. и др. (Mergelov et al.) Почвы и почвоподобные тела оазисов и нунатаков Восточной Антарктиды // Вопросы географии. 2016. Т. 142. С. 593–628.
Никитин Д.А., Марфенина О.Е., Максимова И.А. (Nikitin et al.) Использование сукцессионного подхода при изучении видового состава микроскопических грибов и содержания грибной биомассы в антарктических почвах // Микология и фитопатология. 2017. Т. 51. № 4. С. 211–219.
Дополнительные материалы отсутствуют.
Инструменты
Микология и фитопатология


