Молекулярная биология, 2020, T. 54, № 6, стр. 922-938
Проблемы создания вакцин против бетакоронавирусов: антителозависимое усиление инфекции и вирус Сендай как возможный вакцинный вектор
Т. А. Зайчук a, Ю. Д. Нечипуренко b, *, А. А. Аджубей b, c, С. Б. Оникиенко d, В. А. Черешнев e, С. С. Зайнутдинов f, Г. В. Кочнева f, С. В. Нетесов g, О. В. Матвеева a, h, **
a Sendai Viralytics
117261 Acton, MA, USA
b Институт молекулярной биологии им. В.А. Энгельгардта Российской академии наук
119991 Москва, Россия
c George Washington University
20052 Washington, DC, USA
d Военно-медицинская академия им. С.М. Кирова
194044 Санкт-Петербург, Россия
e Институт иммунологии и физиологии Уральского отделения Российской академии наук
620049 Екатеринбург, Россия
f Государственный научный центр вирусологии и биотехнологии “Вектор”
630559 Новосибирская область, Кольцово, Россия
g Факультет естественных наук Новосибирского государственного университета
630090 Новосибирск, Россия
h Biopolymer Design
117281 Acton, MA, USA
* E-mail: nech99@mail.ru
** E-mail: olga.matveeva@gmail.com
Поступила в редакцию 30.04.2020
После доработки 04.06.2020
Принята к публикации 05.06.2020
Аннотация
Для конструирования вакцины против бетакоронавирусов необходимо использование их эволюционно консервативных антигенных детерминант, на которые у вакцинируемых будет вырабатываться не только гуморальный, но и клеточный иммунный ответ. При разработке вакцин, нацеленных на такие детерминанты, будет минимизирован риск антителозависимого усиления инфекции, которое наблюдали при испытаниях на животных экспериментальных вакцин против таких бетакоронавирусов, как SARS-CoV-1 и MERS-CoV. Эти вакцины были сделаны на основании инактивированного коронавируса или векторных конструктов, экспрессирующих шиповидный белок (S) вириона. Присущая этому белку вариабельность аминокислотных остатков в антигенных детерминантах, в частности рецепторсвязывающего домена (RBD), а также его конформационная изменчивость могут привести к такому же эффекту и при разработке вакцины против SARS-CoV-2. Использование более консервативных структурных и неструктурных вирусных белков для создания вакцины позволит избежать этой проблемы. Одним из них может быть белок нуклеокапсида (N). Согласно данным предварительных исследований, на белок N у выздоровевших пациентов, перенесших COVID-19, вырабатываются антитела в достаточном для нейтрализации вируса титре. В обзоре рассмотрены подходы к разработке вакцин против нового коронавируса ‒ SARS-CoV-2, ‒ которые основаны на векторах не патогенных для человека вирусов. Одним из перспективных вирусных векторов считается вирус Сендай. На его основе возможно создать конструкцию, экспрессирующую целевые антигены (в данном случае SARS-CoV-2). Такая конструкция при интраназальном применении будет индуцировать мукозальный иммунный ответ в слизистой верхних дыхательных путей ‒ “входных воротах” респираторных вирусов, к которым относится и SARS-CoV-2. Именно такой подход будет эффективен для профилактики заболеваний типа COVID-19 и, что важно, позволит избежать антителозависимого усиления SARS-CoV-2-инфекции.
ВВЕДЕНИЕ
Новый бетакоронавирус тяжелого острого респираторного синдрома SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) в конце 2019 года стал причиной вспыхнувшего в Китае инфекционного заболевания, названного COVID-19 (СOronaVirus Infectious Disease), которое затем охватило большинство стран мира и переросло в пандемию. Ожидается, что создание эффективной вакцины, направленной на консервативные антигены бетакоронавирусов, позволит ограничить распространение и предотвратить заболевание COVID-19 или ослабить его течение. Подходы, на основании которых создаются такие вакцины, чрезвычайно разнообразны [1‒5]. В этом обзоре проанализированы проблемы, возникающие при создании коронавирусных вакцин. Кроме того, рассмотрены непатогенные вирусные векторы для экспрессии антигенных детерминант SARS-CoV-2. Особое внимание уделено обоснованию возможного применения векторной системы, основанной на вирусе Сендай, для изготовления вакцин против SARS-CoV-2.
ПРОБЛЕМЫ СОЗДАНИЯ ЭФФЕКТИВНОЙ И БЕЗОПАСНОЙ ВАКЦИНЫ
Серьезной проблемой вакцин против коронавирусов может быть вторичный иммунный ответ, приводящий к антителозависимому усилению инфекции (АЗУИ, или ADE; antibody-dependent enhancement) и развитию респираторного дистресс-синдрома. Желательно как можно раньше выяснить отсутствие у экспериментальной вакцины способности вызывать ADE, хотя эта задача не из простых. Например, эффект ADE выявили при массовой иммунизации детей на Филиппинах вакциной против вируса Денге (Dengvaxia) производства компании “Sanofi Pasteur” (Франция) [6].
Феномен антителозависимого усиления инфекции
Феномен ADE [7‒11] описан для разных вирусов, в том числе и для коронавирусов [12‒16]. На рис. 1 показана схема поглощения комплекса вирус-антитело иммунными клетками в норме и при патологическом развитии инфекции, которое сопровождается ADE. В случае ADE вирусспецифические антитела IgG формируют несовершенные, непрочные комплексы с вирусом, облегчая тем самым инфицирование хозяйских лейкоцитов, несущих рецептор FcγRII [8, 13, 17, 18]. Комплекс антитела с вирусом связывается с FcγRII иммунных клеток и поглощается ими [19]. В норме аффинность вирусспецифических нейтрализующих и одновременно протективных антител гораздо выше, что и приводит к инактивации и последующей элиминации вируса. Дело в том, что вирус, будучи внутри моноцита или макрофага в прочном комплексе с антителом, не может высвободиться и подвергается разрушению. В этом случае происходит выздоровление ‒ что показано в левой части рис. 1. Однако в случае ADE вирус, будучи внутри иммунной клетки в непрочном комплексе, освобождается от антитела и начинает репликативный цикл, как показано в правой части рис. 1.
Рис. 1.
Схема антителозависимого усиления инфекции (ADE) для SARS-CoV-1. Слева показан сценарий правильного иммунного ответа на бетакоронавирус, когда специфические нейтрализующие и протективные антитела способствуют элиминации вируса из организма. Справа представлен сценарий иммунопатологии, которая возникает при изменениях в антигене вируса. ADE наблюдается, когда специфические антитела IgG имеют сниженное сродство к антигену из-за произошедших в нем изменений и поэтому формируют несовершенные комплексы с вирусом. Комплекс антитела с вирусом связывается с рецептором лейкоцитов FcγRII и поглощается этими клетками. Далее, внутри клетки, вирус выходит из эндосомы, уже без антитела, и начинает репликативный цикл [13, 17, 20].
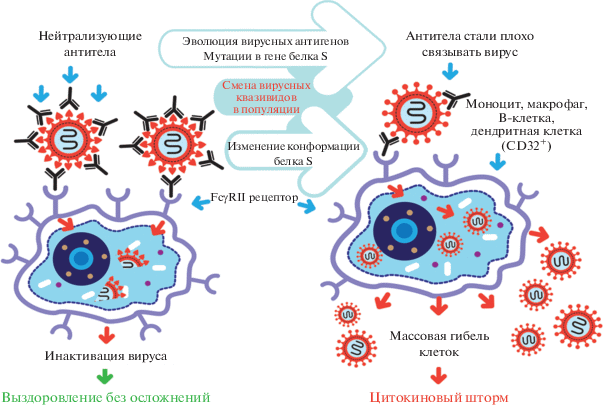
Для SARS-CoV-1 показано, что вирусспецифические антитела к S-белку могут способствовать проникновению вируса в В-клетки [21] и макрофаги [13]. Способность вируса реплицироваться в B-клетках не изучалась. В то же время показано, что в макрофагах вирус начинает реплицироваться, но не дает продуктивной инфекции [13]. Репликация вируса даже без образования инфекционных вирионов может приводить к массовой гибели макрофагов и других иммунных клеток, несущих FcγRII, что будет отягощать течение болезни.
По предварительным данным, SARS-CoV-2 инфицирует CD4+ Т-лимфоциты и, скорее всего, убивает эти клетки [22]. Возможно, что кроме этого процесса, по аналогии с SARS-CoV-1 и MERS-CoV, он инфицирует несущие FcγRII иммунные клетки (такие как моноциты, макрофаги, B-клетки и некоторые виды дендритных клеток) за счет ADЕ.
Антигенная изменчивость шиповидного белка коронавирусов и ADE
На поверхности вириона бетакоронавирусов шиповидный белок (S-белок) образует гомотример, каждая из трех молекул которого состоит из двух субъединиц: S1 и S2 [23]. S1 несет рецепторсвязывающий домен (RBD), а между S1 и S2 находится сайт разрезания сериновыми протеазами. Вирус приобретает способность инфицировать клетки только после того, как происходит протеолитическое расщепление и каждая молекула S-белка разделяется на две субъединицы, которые остаются связанными водородными связями. Каждая из трех субъединиц S1, входящих в тример, может находиться в двух конформациях: открытой и закрытой (рис. 2). В результате исследования структуры S-белка SARS-CoV-2 при помощи криоэлектронной микроскопии показано, что наиболее часто в тримере S-белка одна из субъединиц S1 находится в открытой конформации, а две другие ‒ в закрытой [23].
Рис. 2.
Конформации S-белка в составе гомотримера. Все субъединицы S1 находятся в закрытой конформации (а), одна субъединица находится в открытой конформации, две в закрытой (б). в ‒ Схематично показана доменная структура и конформации S-белка в составе тримера. Рецепторсвязывающий домен (RBD, голубой) вместе с N-концевым доменом (желтый) входит в состав субъединицы S1 белка S; между субъединицей S1 и S2 (красно-коричневая) находится сайт протеолитического расщепления для фурина, а внутри субъединицы S2 находится сайт расщепления протеазой TMPRSS2 [24]. Слева – открытая конформация, справа – две закрытых. Внизу ‒ доменная структура молекулы S-белка. Изображения взяты из базы PDB [25].
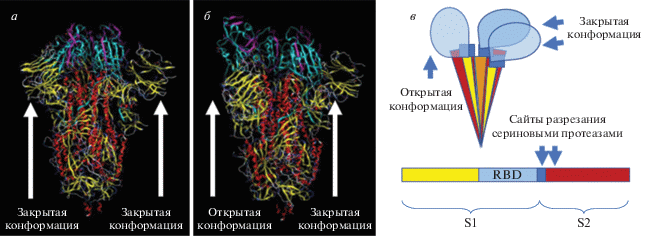
Как видно из рис. 2, при изменении конформации S-белка его антигенные детерминанты тоже будут претерпевать перестройки. Таким образом, причиной феномена ADE у бетакоронавирусов может быть антигенная изменчивость S-белка, обусловленная не только вариабельностью аминокислотной последовательности [26], но и ее конформационной подвижностью [27, 28], что приводит к перестройкам в антигенных детерминантах.
Механизм ADE у бетакоронавирусов изучен на примере меняющихся иммунодоминантных детерминант S-белка вирусов SARS-CoV-1 и MERS-CoV [14, 16]. У вирусов SARS-CoV-1 и SARS-CoV-2 этот домен высокогомологичен и отвечает за связывание одного и того же мембранного клеточного рецептора ‒ ангиотензинконвертирующего фермента-2 (ACE2; angiotensin converting enzyme 2), который служит “входными воротами” в клетку для обоих вирусов [29].
Недавно Wan и соавт. [16] описали механизм ADE, индуцируемый MERS-CoV. Они показали, что моноклональные нейтрализующие антитела, специфичные к RBD, опосредуют проникновение вируса в иммунные клетки.
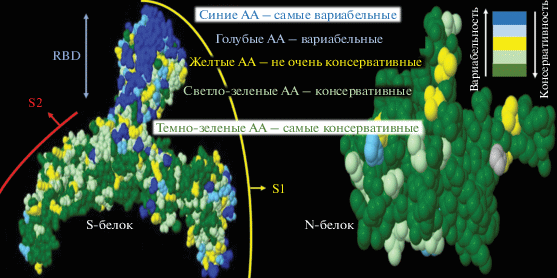
На основании проведенного анализа литературы и геномных последовательностей вирусов Ricke и соавт. [26] выдвинули гипотезу, что для заражения иммунных клеток вирусы SARS-CoV-1, MERS-CoV и SARS-CoV-2 используют универсальный механизм, приводящий к ADE. Проведенный авторами анализ мутационной изменчивости белков SARS-CoV-2 нам представляется очень важным в этом контексте (см. рис. 3).
Рис. 3.
Модели белков S и N SARS-CoV-2. Консервативные и вариабельные аминокислоты (АА) показаны разным цветом. Модели белков воспроизведены из препринта [26]. Предполагается, что высокая вариабельность S-белка обусловлена его экспонированностью на вирусной оболочке и вследствие этого ускоренным антигенным дрейфом под давлением иммунного отбора, в отличие от белка N, в основном находящегося внутри вириона [26]. Часть молекулы белка N экспонирована на поверхности вирионе и на нее вырабатываются нейтрализующие и протективные антитела [30].
Иммунодоминантные эпитопы в S-белке SARS-CoV-2 формируют аминокислотные остатки в позициях от 441 до 700 [31]. В изолятах SARS-CoV-2 в позиции 614 S-белка часто находят либо аспарагиновую кислоту, либо глицин (614D/G). Некоторые исследователи связывают замену в этой позиции со способностью вируса вызывать ADE [32]. На сайте Национального Центра Биоинформатики Китая (China National Center for Bioinformation) [33] приведена модель трехмерной структуры S-белка и на ней указано положение аминокислотного остатка D614, который находится на границе участков β-структуры и спирали типа полипролин II (согласно классификации вторичных структур белков [34, 35]).
Позиция 614 находится на некотором расстоянии от RBD, однако замена отрицательно заряженной аспарагиновой кислоты на нейтральный глицин может играть ключевую роль в возникновении ADE из-за изменения антигенных детерминант [32, 36]. Korber и соавт. [32] для объяснения этого феномена предложили две гипотезы. Первая заключается в том, что замена аминокислоты может вызывать ADE напрямую, что обусловлено изменением константы связывания антител с антигеном, в состав которого входит аминокислотный остаток 614. Аргументация авторов основана на том, что у SARS-CoV-1 антитела к пептиду LYQDVNC вызывают ADE. Этот 7-членный пептид идентичен в S-белках SARS-CoV-1 (597–603) и SARS-CoV-2 (611‒617). Согласно второй гипотезе, замена аминокислоты в позиции 614 может приводить к ADE, инициируя переход открытой конформации S-белка в закрытую. Такой конформационный переход может быть связан с ослаблением взаимодействия между субъединицами S1 и S2 из разных молекул S-белка в составе гомотримера. Именно это и происходит при замене заряженной аспарагиновой кислоты на нейтральный глицин в позиции 614 ‒ исчезает водородная связь между субъединицами S1 и S2.
Кроме того, ADE может быть обусловлено изменением сайтов гликозилирования в молекуле S-белка. Watanabe и соавт. [37] идентифицировали 22 сайта потенциального гликозилирования в S-белке SARS-CoV-2. С одной стороны, известно, что углеводные цепи белков затрудняют связывание антител с эпитопами и тем самым способствуют образованию низкоаффинных комплексов антитело-вирус, то есть “работают” на ADE. С другой стороны, сами углеводные цепи могут входить в состав антигенных детерминант, а их отсутствие в составе белка будет опять-таки приводить к снижению аффинности связывания антитела с антигеном – и это снова “игра в одни ворота”.
Заражение нового хозяина в действительности происходит при проникновении в его организм не одного варианта вируса, а целой популяции генетически близкородственных вариантов, возникших в результате мутаций, приобретенных в ходе инфекционного цикла вируса в предыдущих хозяевах [38]. Такая популяция получила название квазивида. Концепция квазивидов очень важна для понимания механизма ускользания вируса от иммунного ответа хозяина. Одни варианты квазивидов вируса могут быть нейтрализованы антителами нового хозяина, а другие, к которым у антител низкое сродство, могут получить эволюционное преимущество и стать драйверами ADE.
Все эти факторы приходится учитывать при разработке вакцин (и не только против коронавирусов). Их недооценка может привести к тому, что созданная на основании только одного варианта белка (скажем, S-белка коронавируса) вакцина будет индуцировать выработку антител с низкой аффинностью к циркулирующему штамму, уже претерпевшему антигенный дрейф или недавно появившемуся и потому антигенно отличному от вакцинного вируса. В результате комплекс антитело-вирус берет на себя роль “троянского коня”: облегчает вирусу проникновение в хозяйские моноциты или макрофаги, а высвободившийся вирус запускает в этих клетках инфекционный процесс. Такой же сценарий возможен при первичной инфекции в процессе ее развития в организме из-за изменения вирусных эпитопов под действием иммунного ответа хозяина. Возможен он и при вторичной инфекции мутировавшим или близкородственным вирусом после уже перенесенного заболевания [39].
ADE и респираторный дистресс-синдром
Во многих лабораториях, изучающих на животных моделях болезни, вызванные SARS-CoV-1 и MERS-CoV, наблюдали значительные повреждения легких, по мнению авторов ассоциированные с ADE [12‒16, 40]. Кроме того, уже известны случаи опосредованного макрофагами тяжелого повреждения легких у человека и приматов, при этом в механизм патогенеза вовлечены антитела класса IgG, специфичные к S-белку SARS-CoV-1 [41]. На кроликах, использованных в качестве животной модели заболевания, вызываемого MERS-CоV, показано, что ADE может развиваться и при повторном заражении. Так, у животных, интраназально инфицированных MERS-CоV, развивалась легочная патология, характеризующаяся виремией и воспалением легких тяжелой степени. При повторном заражении MERS-CоV, несмотря на наличие антител, кролики заболевали снова и повреждения легких были более тяжелыми, чем при первичной инфекции [41]. Инфекция вирусами SARS-CoV-1 [42] или MERS-CoV [43] вызывала более тяжелую пневмонию у вакцинированных животных, несмотря на высокий уровень специфических нейтрализующих антител. Интересно, что при исследовании эффективности вакцинации инактивированным вирусом на животных моделях получены аналогичные, то есть негативные, результаты [44].
Следует отметить, что иногда разработчики оценивают эффективность вакцины исключительно по ее способности индуцировать продукцию протективных антител, не вызывая серьезных патологий во внутренних органах животного через 3 суток после инфицирования вирусом [45]. Оценка безопасности вакцины по этим критериям недостаточна, так как не позволяет анализировать синдром повреждения легких, который может возникнуть через 4‒10 суток после инфекции из-за развития ADE [45]. К серьезным недостаткам оценки безопасности вакцины на животных моделях относится маленький размер групп испытуемых животных. Иногда общий вывод о безопасности вакцины делали при изучении экспериментальной и контрольной групп всего с тремя животными в каждой [21].
Некоторые исследователи уже продемонстрировали отсутствие спровоцированного вакцинацией эффекта ADE на животных моделях и в клеточных культурах (см. например [46]). Используя вирусоподобные частицы, сделанные на основании S-белка SARS-CoV-2, экспрессированного в ретровирусном конструкте, авторы показали, что сыворотка животных после иммунизации рекомбинантным RBD-фрагментом S-белка препятствует проникновению вирусоподобных частиц в клетки, экспрессирующие антителосвязывающие рецепторы макрофагов и моноцитов ‒ FcγRII (CD32). Следует сказать, что в этой работе естественный процесс вирусной инфекции смоделирован лишь частично, так как не воспроизводит всего разнообразия природных последовательностей и конформаций S-белка SARS-CoV-2.
Феномен ADE, опосредованный антителами к полноразмерному S-белку SARS-CoV-1, наблюдали у приматов. Несмотря на то, что вакцинация снижала вирусную нагрузку при последующем заражении SARS-CoV-1, наличие IgG-антител к S-белку у иммунизированных макак значительно усиливало воспалительное повреждение легких [41]. У людей иммунодоминантный эпитоп S-белка SARS-CoV-1 индуцировал продукцию как нейтрализующих антител, так и антител, усиливающих инфекцию [41].
ADE как возможная причина патогенеза SARS и COVID-19
По мнению ряда исследователей (Ricke и соавт. [26], Korber и соавт. [32], Нечипуренко с соавт. [36]), в основе патогенеза серьезных коронавирусных заболеваний, SARS и COVID-19, может лежать механизм ADE. Предполагается, что инфицирование макрофагов и моноцитов ‒ ключевой этап в развитии болезни и ее прогрессирования от легкой формы к тяжелой. ADE влечет за собой дисфункцию иммунной системы организма. Это и апоптоз иммунных клеток, приводящий к развитию Т-клеточной лимфопении, и воспалительный каскад с накоплением макрофагов, цитокинов и хемокинов в легких, и цитокиновый шторм [26].
Jaume и др. показали [20], что в инфицированных SARS-CoV-1 клетках иммунной системы человека экспрессия двух видов рецепторов: FcγRIIa и FcγRIIb (но не FcγRI или FcγRIIIa) ‒ индуцирует ADE. Yuan и соавт. [47], наблюдая за больными SARS, обнаружили, что тяжесть заболевания коррелирует с аллельным полиморфизмом FcγRIIa: при изоформе FcγRIIa, взаимодействующей и с IgG1, и с IgG2, болезнь протекала тяжелее, чем при изоформе FcγRIIa, связывающей только IgG2 [47].
Возможно, именно механизм ADE вовлечен в патогенез тяжелых форм заболевания COVID-19, которые превалируют среди возрастных пациентов [36]. В результате иммуностарения у пожилых людей выработка антител идет медленнее, чем у молодых, поэтому к тому времени, когда титр антител достигает уровня, необходимого для нейтрализации вируса, антигенные детерминанты патогена успевают измениться. Это может происходить вследствие либо прямых мутаций, либо активации квазивида, отличного от первоначального. В этом случае антитела образуют с вирусом нестабильные комплексы и “затаскивают” живой вирус в моноциты и макрофаги, где тот реплицируется; в результате развивается генерализованная инфекция и цитокиновый шторм. В подтверждение этой гипотезы можно привести тот факт, что в сыворотке госпитализированных пациентов, вошедших в исследование, уровень IgG-антител к S-белку SARS-CoV-2 коррелировал с возрастом и тяжестью заболевания: чем выше титр, тем старше пациент и тем тяжелее протекала болезнь [48]. Кроме того, выявлена положительная и значимая корреляция между уровнем антител в крови и концентрацией маркера воспаления ‒ C-реактивного белка [48].
Известны и другие факты, подтверждающие гипотезу о возможном “вреде” некоторых вариантов антител к S-белку. В результате сравнительного анализа специфического гуморального ответа больных SARS было выявлено, что на 15-й день заболевания у пациентов, впоследствии умерших, титр антител к S-белку был значимо выше, чем у тех, кто впоследствии выздоровел [49].
Антигенный импринтинг и патогенез COVID-19
Другая гипотеза, выдвинутая J. Tetro [50], связана с явлением антигенного импринтинга [7, 10, 51, 52], в основе которого лежит способность организма при первичной инфекции образовывать долгоживущие клетки иммунологической памяти ‒ они остаются в организме и обеспечивают защиту от последующих инфекций близкородственными патогенами. Гипотеза основана на возможной иммунологичной кросс-реактивности между сезонными низкопатогенными коронавирусами и SARS-CoV-2. Клетки иммунологической памяти (если они представлены В-клетками) реагируют на специфические эпитопы на поверхности вирусов для производства антигенспецифических антител. Реакция В-клеток памяти на новую инфекцию, но “старые” антигены происходит быстрее по сравнению с реакцией наивных В-клеток, которые начинают вырабатывают антитела уже на новые антигены [52].
Антигенный импринтинг ускоряет иммунный ответ. В этом его основная роль для эффективной и быстрой борьбы с инфекцией. Однако у этого явления есть и негативная сторона. Она может реализоваться тогда, когда новый вирус предпочтительно реактивирует В-клетки памяти, которые появились в результате заболевания сезонными низкопатогенными родственными вирусами (в рассматриваемом случае коронавирусами), и стимулирует выработку соответствующих антител. Не исключено, что такие антитела будут иметь пониженное сродство к эпитопам нового вируса и поэтому стимулировать слабый иммунный ответ и/или индуцировать ADE. Как следствие ADE может наступить массовая гибель иммунных клеток, причем этот процесс развивается очень быстро и на ранних этапах инфекции ‒ еще до того, как организм начнет производить достаточное количество вирусспецифических протективных антител. Так как с возрастом пул B-клеток памяти, способных вырабатывать антитела к “пережитым инфекциям”, в том числе сезонным и низкопатогенным коронавирусам, скорее всего, увеличивается, то причиной того, что у пожилых людей COVID-19 протекает в тяжелой форме, может быть антигенный импринтинг. В то же время нельзя исключить, что антигенный импринтинг, выработанный на сезонные коронавирусы, может предотвращать или облегчать течение COVID-19 ‒ благодаря быстрой реакции иммунной системы на патоген. Скорее всего, развитие заболевания в той или иной форме зависит как от индивидуальных особенностей всех систем организма человека, так и от репертуара патогенов, с которыми его иммунная система уже сталкивалась.
ИММУННЫЙ ОТВЕТ У БОЛЬНЫХ COVID-19
Гуморальный иммунный ответ
Long и соавт. [53], анализируя иммунный ответ переболевших COVID-19, обнаружили, что уровень антител IgG, как у бессимптомных вирусоносителей, так и у перенесших симптоматическую форму, снижался в среднем на 70% уже через 8 недель после полного выздоровления, при этом у многих до недектируемого уровня. А Jiang и др. [54] показали, что у заболевших COVID-19 антитела IgG и IgM вырабатываются прежде всего на S-белок SARS-CoV-2 (в частности субъединицу S1). В меньшей степени антитела переболевших узнают белок N, протеазу NSP5 и белок ORF9b. Это согласуется и с данными Lu и соавт. [31] по идентификации иммунодоминантных эпитопов в S-белке SARS-CoV-1.
Учитывая, что в составе вириона S-белок находится на его поверхности в виде гомотримера, в котором субъединица S1 экспонирована гораздо больше, чем S2 [55], логично предположить, что антитела, узнающие эпитопы на S1, нейтрализуют вирус, в то время как для антител, которые нацелены на S2 и белки, находящиеся внутри вириона, это маловероятно. Тем не менее показано, что концентрация антител против антигенных детерминант белка N в крови людей, переболевших COVID-19, коррелирует с титром нейтрализующих антител к вирусу SARS-CoV-2 [30].
Антигенные детерминанты S-белка изменчивы и в процессе эволюции коронавируса их перестают узнавать антитела, выработанные на ранее циркулировавший антигенный вариант. В широкомасштабном исследовании сывороток пациентов, переболевших COVID-19, подтверждена быстрая антигенная изменчивость S-белка и показано, что антитела на S-белок SARS-CoV-2 не связываются с гомологичным S-белком SARS-CoV-1 [56]. Не исключено, что иммунологическая память В-клеток, вырабатывающих антитела на S-белок, сравнительно кратковременна. Учитывая, что по представленности эпитопов на поверхности вириона S-белок доминирует среди других белков коронавируса, логично предположить, что и иммунологическая память В-клеток на бетакоронавирусы в целом также кратковременна.
На основании вышеизложенного можно предположить, что антитела, выработанные на вакцинные антигены S-белка, не защитят от естественного инфицирования вирусом, эпитопы которого изменились вследствие антигенного дрейфа. В то же время антигенные детерминанты N-белка, скорее всего, гораздо меньше подвержены антигенному дрейфу (см. рис. 3), поэтому риск ADE для антител, выработанных на такие детерминанты, гораздо ниже, чем для антител к S-белку. Увы, пока неизвестно, могут ли антитела к N-белку иметь протективную значимость.
Т-клеточный иммунный ответ
T-клеточный иммунный ответ чрезвычайно важен для эффективного подавления инфекции. Не исключено, что этот ответ, выработанный на консервативные антигенные детерминанты сезонных (обычно респираторных) коронавирусов, способен предотвращать или ослаблять инфекцию SARS-CoV-2 [57]. Это предположение подтверждается результатами Grifoni и соавт. [58], которые обнаружили T-клетки CD4+ и CD8+, узнающие антигенные эпитопы SARS-CoV-2, у половины из двух десятков здоровых доноров, кровь которых была собрана до эпидеми COVID-19. Среди узнаваемых эпитопов были петиды, входящие в состав белков N, M, NSP3, NSP4, ORF3a и ORF8 коронавирусов; причем эффективность Т-клеточного иммунного ответа на эти эпитопы у больных COVID-19 не уступала ответу на эпитопы S-белка.
Уже появились работы [26, 59, 60], где на основании биоинформационного анализа экспериментальных данных идентифицированы эволюционно консервативные линейные антигенные детерминанты SARS-CoV-2, которые можно использовать при разработке вакцины, индуцирующей Т-клеточный иммунный ответ. Среди этих детерминант ‒ пептиды белков N, M и Е [59].
Таким образом, выяснено, что у SARS-CoV-2 есть консервантивные антигенные детерминанты для индукции Т-клеточного иммунного ответа и их картирование станет отправной точкой для конструирования вакцины, индуцирующей Т-клеточный иммунный ответ на этот патоген.
Мукозальный иммунный ответ в респираторном тракте
Показано, что SARS-CoV-2 хорошо реплицируется в клетках, которые экспрессируют ACE2 ‒ рецептор S-белка вируса ‒ и сериновые протеазы фурин и TMPRSS2, которые разрезают S-белок и тем самым активируют вирус [61]. А значит хозяевами вируса, прежде всего, могут быть клетки, экспрессирующие ACE2, фурин и TMPRSS2. Это бокаловидные (goblet) клетки в полости носа, секреторные клетки транзиентного типа, находящиеся между бокаловидными и реснитчатыми клетками; пневмоциты первого и второго типа, которые поддерживают альвеолы; и энтероциты в тонком кишечнике, отвечающие за адсорбцию питательных веществ [61‒63].
Появились данные, на основании которых можно сделать вывод о возможности распространения вируса, минуя рецептор, ‒ путем слияния инфицированных клеток с незараженными клетками, что происходит под действием S-белка [64]. Такой механизм распространения инфекции приводит к образованию многоядерных клеток-синцитиев. Слияние клеток и образование синцитиев облегчает распространение вируса от клетки к клетке и его ускользание от иммунного ответа хозяина [64]. Также теоретически возможно и заражение иммуных клеток по механизму ADE. Такое заражение может быть основным механизмом патогенеза COVID-19.
Следует сказать, что у некоторых пациентов с COVID-19 заболевание сопровождается диареей [65]. Пока не понят механизм инфицирования энтероцитов кишечника коронавирусом. Учитывая, что при COVID-19 диарея встречается редко, можно предположить, что основными “входными воротами” вируса SARS-CoV-2 в организм служат все-таки клетки респираторного тракта, и значит именно на них следует “делать ставку” при разработке противовирусных и профилактических средств.
Принимая во внимание, что SARS-CoV-2 может распространяться от клетки к клетке, не выходя в межклеточное пространство и вызывая образование синцития, можно предположить, что его встреча с антителами при выходе вируса в кровяное русло происходит с запозданием ‒ возможно, только на поздних этапах инфекции. В связи с этим желательно, чтобы вакцина предотвращала попадание вируса в организм через основные “входные ворота”, находящиеся в эпителии слизистой оболочки верхних дыхательных путей, прежде чем он окажется в крови. Крайне важно, чтобы вакцина индуцировала мукозальный иммунитет, то есть продукцию прежде всего антител IgA [66]. Стоит заметить, что присутствия протективных антител в крови, которые могут образоваться после вакцинации, даже с высоким титром, может быть недостаточно для инактивации вируса именно в слизистой оболочке эпителия верхних дыхательных путей и именно на ранних этапах инфекции [16].
Для респираторынх патогенов создание вакцины, которая предотвращает инфекцию в первичном очаге, ‒ труднейшая задача, но именно такая вакцина будет максимально эффективна в случае SARS-CoV-2.
ВЕКТОРНЫЕ ВАКЦИНЫ
Один из современных подходов к разработке вакцин заключается в экспрессии антигенных детерминант белков патогена в векторном конструкте на основе непатогенного для человека вируса. Преимущество этого подхода ‒ в высокой иммуногенности и возможности индуцировать как клеточный, так и гуморальный иммунитет против патогена. Следует отметить, что вакцины, созданные на основании этого подхода, пока прошли только первую стадию клинических испытаний [67].
Учитывая, что векторный вирус в организме человека проходит ограниченный репликативный цикл без развития инфекции, понятно, что к нему предъявляются определенные требования: способность к экспрессии встроенных в него трансгенов (генов патогена) в клетках человека и безопасность для человека. Такие векторные вирусы уже известны. Вот некоторые из них: вакцинный вирус кори, вирус осповакцины, аденовирусы, аденоассоциированные вирусы и другие [68, 69].
Китай одним из первых начал разработку вакцин против COVID-19. Компания “CanSino Biological Inc.” (Китай) в сотрудничестве с Институтом биотехнологии Пекина (Beijing Institute of Biotechnology) разрабатывает вакцину на основании аденовируса серотипа 5 (AdV5). Ряд других компаний также использует в качестве вектора AdV5 для создания вакцин против COVID-19. Например, крупная фармацевтическая компания “AstraZeneca” (Великобритания) совместно с Оксфордским университетом (Oxford University, Великобритания) приступила к третьей фазе клинических испытаний. До этого были проведены испытания этой вакцины на животной модели приматов [70]. Однoкратное введение дозы вакцинного материала индуцировало у животных выработку антител, но не обеспечивало 100%-ную защиту от заражения. В то же время вакцинированные обезьяны переносили экспериментальную инфекцию в гораздо более легкой форме по сравнению с контрольными, у которых после заражения развивалась вирусная пневмония.
Следует сказать, что использование AdV5 в качестве вакцинного вектора достаточно проблематично. Дело в том, что в человеческой популяции уже есть антитела к AdV5 [71]; это так называемые предсуществующие антитела, которые будут снижать эффективность вакцинации.
Несколько организаций занимается разработкой вакцины, используя в качестве вектора вакцинный штамм вируса кори. Это Институт Пастера (Institute Pasteur) во Франции в сотрудничестве с центром Питтсбурга по вакцинным исследованиям (Pittsburg Center for Vaccine Research) в США и Университетом Темиса в Австралии (Temis University), а также компания “Zydus Cadila”, которая находится в Индии [72]. Подобный подход выбрали и российские ученые из Института общей генетики РАН [73]. Аттенуированный вирус кори как векторная основа уже успешно использован учеными из Германии для создания экспериментальной вакцины против MERS [74].
Интересен подход ученых из Китая, которые используют в качестве векторной основы вирус парагриппа 5 [75]. Этот вирус, как и вирус кори, относится к семейству Paramyxoviridae. У него есть и другие названия: вирус парагриппа обезьян 5 и Simian virus 5 [76]. Вирус парагриппа 5 не вызывает заболевания у человека, поэтому в человеческой популяции антитела к нему, скорее всего, отсутствуют. Это значит, что вирус парагриппа 5 можно рассматривать в качестве потенциального вектора для вакцин.
Теоретически вакцину против COVID-19 можно разработать и на основе другого парамиксовируса ‒ вируса болезни Ньюкасла, который вызывает тяжелое заболевание у птиц, но сравнительно легкий конъюнктивит и фарингит у человека [77‒79].
Стоит обратить внимание на еще один подход к созданию вакцины против коронавируса, который разработан учеными из Китая и уже проходит клинические испытания, ‒ вакцинация конструктами на основе лентивирусных векторов, которые экспрессируют минигены, кодирующие антигены SARS-CoV-2 [80].
Вирус Сендай как векторная основа вакцины
Вирус Сендай (Murine respirovirus) считается перспективным вектором для создания вакцины против SARS-CoV-2. Основное преимущество этого вируса перед другими кандидатами в вирусные векторы состоит в том, что он реплицируется в клетках бронхиального эпителия человека, не вызывая заболевания [81], а также в некоторых типах дендритных клеток [82]. Показано, что вирус Сендай размножается в клетках бронхов приматов, не вызывая заболевания [83]. Таким образом, этот вирус может быть использован для доставки вирусных антигенов в клетки бронхиального эпителия и в дендритные клетки человека.
Как упоминалось выше, для респираторных патогенов, к которым относится и SARS-CoV-2, с помощью вакцины крайне важно остановить инфекцию в слизистой эпителия верхних дыхательных путей (см. раздел “Мукозальный иммунный ответ в респираторном тракте”). Следовательно, необходимо, чтобы вакцина стимулировала выработку IgA-антител. Именно поэтому вектор, основанный на вирусе, который сам относится к респираторным патогенам млекопитающих, но не вызывает заболевания у человека, лучше всего подходит для этой цели.
Вирус Сендай вызывает инфекционное заболевание только у грызунов и известен научному сообществу почти 70 лет. Все это время его активно использовали как модельный парамиксовирус в молекулярно-биологических исследованиях. Кроме того, показано и его онколитическое действие на некоторые опухоли [84]. Несмотря на длительное лабораторное использование, до сих пор не зарегистрировано ни одного случая заболевания у человека или домашних животных. Более того, безопасность вируса Сендай доказана в клинических испытаниях его иммуногенности в отношении вируса парагриппа 1 человека. Живой вирус Сендай в виде капель вводили интраназально взрослым и детям. Эта процедура не вызывала серьезных побочных эффектов ни у взрослых, ни у детей и индуцировала образование антител, перекрестно взаимодействующих с вирусом парагриппа 1 человека [85, 86]. Результаты этих испытаний стали доказательством безопасности вируса Сендай для человека, а также его способности быть эффективным респираторным иммуногеном.
Еще одно важное преимущество вируса Сендай как вектора для вакцины к коронавирусу состоит в том, что вакцинный материал, содержащий вирусный конструкт, можно вводить интраназально [85, 86]. Этот тип доставки вирусных антигенов для вакцинации обеспечивает долговременную защиту слизистой оболочки дыхательных путей от патогена и особенно эффективен в отношении вирусов, вызывающих респираторные инфекции, в том числе SARS-CoV-2. Интересно, что интраназальная вакцинация вектором вируса парагриппа 5, экспрессирующим иммуногены MERS-CoV, оказалась гораздо эффективнее внутримышечной в предотвращении инфекции у экспериментальных животных [75].
Более того, интраназальное введение, по сравнению с внутримышечными инъекциями, более эффективно в преодолении потенциально предсуществующего иммунитета к вирусу Сендай [87]. Этот предсуществующий иммунитет выражается в присутствии у многих людей антител к вирусу парагриппа 1 человека, которые перекрестно реагируют с вирусом Сендай. В исследовании Hara и соавт. [88], опубликованном в 2011 году, продемонстрировано, что специфические нейтрализующие вирус Сендай антитела IgG (образовавшиеся вследствие перенесенной инфекции, вызванной парагриппом 1), можно выявить у 93% всей человеческой популяции. Тем не менее Hara и соавт. [88] считают, что такие антитела не стоит рассматривать как препятствие для создания векторных вакцин на основе вируса Сендай, так как их титр у большинства людей не достигает того уровня, который влияет на способность вакцины стимулировать гуморальный и антигенспецифический Т-клеточный иммунный ответ [89].
В доклинических исследованиях [90] и в клинических испытаниях [67] показано, что вирус Сендай индуцирует продукцию антигенспецифичных Т-киллерных клеток. Разработка вакцины против ВИЧ-инфекции с вектором, сконструированным на основе вируса Сендай, находится на втором этапе клинических испытаний. Эта вакцина включает в себя ген, кодирующий белок Gag ВИЧ-1. У людей она вызывает мощную индукцию Т-клеточного ответа и продукцию специфических нейтрализующих антител к белку Gag в режиме прайм-буст [67, 90]. Вирус Сендай также использовали в качестве основы при разработке вакцины против туберкулеза [91, 92] и вакцины против респираторно-синцитиального вируса [93, 94], которая проходит первую фазу клинических испытаний [93].
Очень важный фактор ‒ генетическая стабильность вируса Сендай, который, как и другие представители семейства Paramyxoviradae, эволюционирует очень медленно [95]. Как и геномы других парамиксовирусов, вирус Сендай содержит 6 генов, которые кодируют 6 основных белков. Таким образом, этот вирус относится к тем, которые регулируются “правилом шести” [96‒98]: в вирусе с полигексамерным геномом (6n + 0) затруднена гомологичная рекомбинация, что обеспечивает его высокую стабильность.
Геном вируса Сендай представляет собой несегментированную минус-цепь РНК длиной 15 384 нуклеотидов и содержит гены, кодирующих белки поверхностных гликопротеинов HN (гемагглютинин-нейраминидаза) и F (белок слияния), нуклеокапсидные белки NP (белок нуклеокапсида), P (малая субъединица РНК-полимеразы, или фосфопротеин) и L (большая субъединица РНК-полимеразы), а также внутренний матриксный белок М [95, 96]. Последовательность генов вируса Сендай представлена на рис. 4.
Рис. 4.
Схема организации геномов вирусов SARS-CoV-2 и Сендай. Вверху ‒ геном вируса SARS-CoV-2, расположение кодируемых белков и вариабельность некоторых аминокислот этих белков в виде гистограммы. Шкала вариабельности показывает пропорцию неидентичных аминокислот в каждой позиции по базе данных, собранных в период с декабря 2019 года до июня 2020 года [99] и включающей 2 921 вирусный вариант. За эталон принята первая размещенная в базе данных аминокислотная последовательность белков, кодируемых геномом SARS-CoV-2. Вариабельность аминокислоты в каждой позиции рассчитывали как долю встречающихся в базе данных аминокислот, не идентичных эталонной, для всех белков в транслируемой части генома. Внизу ‒ геном вируса Сендай и потенциальные сайты интродукции трансгенов [59, 60]. Трансгены могут кодировать консервативные антигенные детерминанты SARS-CoV-2 [30, 54, 100]. Латинскими буквами указаны кодируемые белки. Сверху над схемой генома вируса Сендай цифрами указана длина отдельных генов, а снизу ‒ размер соответствующих белков.
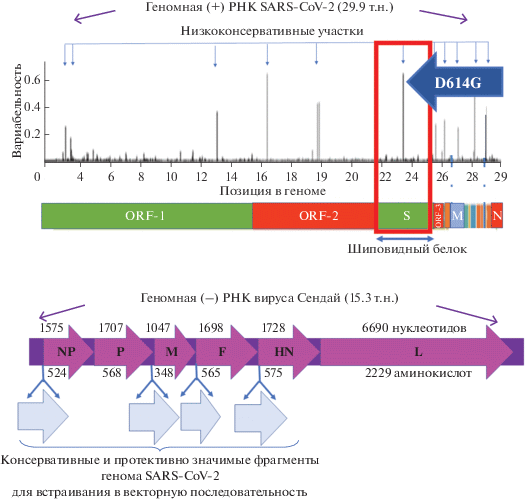
Сравнительно простая структура генома вируса Сендай за последние два десятка лет вдохновляла многих исследователей на создание разнообразных генетических конструкций с использованием его в качестве векторной основы. Благодаря тому, что репликация вируса происходит исключительно в цитоплазме, а не в ядре, риск генетической интеграции вирусного генома в геном хозяина минимален. Такие конструкты создают путем встраивания целевых генов в межгенные области вируса Сендай или вместо вирусных генов F, HN и M [86, 90, 91, 93, 94, 101‒104]. Показано, что трансгены размером более 3 т.п.н. могут быть встроены и экспрессированы в геноме вируса Сендай [103]. К оптимальным районам для встраивания протяженных генетических элементов относятся 3'-некодирующая лидерная последовательность, расположенная перед геном NP [105], области между генами F и M [106] и между генами F и HN [107], а также 3'‑некодирующая область гена P [108]. Таким образом, создание генетической конструкции на основе вируса Сендай, экспрессирующей гены коронавируса SARS-CoV-2, теоретически возможно.
К несомненным преимуществам использования вируса Сендай в качестве вакцинного вектора относится и его способность эффективно реплицироваться в энтодермальных клетках и накапливаться в аллантоисной полости куриных эмбрионов [109]. Кроме того, вирус Сендай можно адаптировать к росту в клеточных линиях млекопитающих. Множественные циклы направленного отбора увеличивают титр вируса Сендай в различных клеточных культурах [110‒112].
Наличие надежной животной модели для испытания вакцин ‒ чрезвычайно важный фактор. Одна из проблем при использовании вируса Сендай в качестве вектора может возникнуть на стадии доклинических испытаний вакцины. Дело в том, что широко используемая в этих случаях мышиная модель не всегда подходит для вируса Сендай из-за чувствительности мышей к этой инфекции. Однако некоторые линии, такие как C57Bl/6J, относительно устойчивы к заражению и могут переносить высокие инфекционные дозы вируса [102, 113]. Крысы линии F344 также резистентны к вирусу Сендай [114].
На сегодняшний день появились сообщения о трансгенной линии мышей, чувствительной к инфекции SARS-CoV-2 [48]. Ранее Gretebeck и др. [115] разработали мышиную модель для изучения инфекции MERS, на которой показали, что у молодых мышей, в отличие от старых, несмотря на детектируемую репликацию вируса в легких, не развиваются клинические признаки заболевания. Однако уровня репликации вируса в дыхательных путях молодых мышей, даже без каких-либо признаков заболевания, может быть достаточно для оценки эффективности вакцины против SARS-CoV-1 [116]. Эти наблюдения позволяют предположить сходную восприимчивость дыхательных путей мышей или крыс к репликации вируса SARS-CoV-2 после циклов адаптации ‒ тогда гуморальные и клеточные иммунные ответы на кандидатную вакцину можно будет оценить на этих животных моделях.
Сотрудники университета Фудан в г. Шанхай (Fudan University, Китай) вместе с коммерческими партнерами занимаются разработкой вакцины для профилактики COVID-19, используя репликативно некомпетентный вариант вируса Сендай [117, 118]. Репликативно компетентный векторный конструкт на основе вируса Сендай также считается перспективным направлением в разработке вакцин [67, 85, 86]. Оба типа векторов, способные и не способные к репликации, имеют свои плюсы и минусы. На основании досконального анализа вышеупомянутых исследований мы пришли к выводу, что иммуногенность репликативно компетентного вектора выше, чем нереплицирующегося, а его получение включает меньше стадий и потому при крупномасштабном производстве будет более рентабельным.
ЗАКЛЮЧЕНИЕ
Мы предполагаем, что вирусное заражение иммунных клеток SARS-CoV-2 по механизму ADE может реализоваться как при тяжелом течения болезни после первичного заражения вирусом, так и при повторном заражении уже антигенно измененным штаммом. Иммунизация против SARS-CoV-2 может усугубить последующую инфекцию и этот факт необходимо учитывать, как при изучении патогенеза COVID-19, так и при прогнозировании следующих волн эпидемии. Привлечение внимания к явлению ADE, его механизму и возможностям моделирования очень актуально сейчас, когда уже начинаются испытания вакцин против COVID-19.
Можно надеяться, что использование в вакцине против SARS-CoV-2 консервативных антигенных детерминант N-белка вместо быстро изменяющихся детерминант S-белка позволит добиться, во-первых, долговременного иммунного ответа на этот патоген и, во-вторых, снижения вероятности развития ADE. Уже показано, что белок N вируса эволюционно гораздо более консервативен, чем S [59]; причем, согласно данным предварительных исследований, на консервативные антигенные детерминанты N-белка у больных вырабатываются антитела в достаточном для нейтрализации вируса титре [30].
Все гены белков, кодирующих консервативные антигенные детерминанты вируса, могут быть включены в конструкты, основанные на векторной платформе вируса Сендай.
Векторная платформа на основе этого вируса обладает рядом преимуществ. Вирус характеризуется высокой геномной стабильностью и безо-пасностью для человека. Кроме того, вакцины, сконструированные на основе респираторных вирусов, к категории которых относится и вирус Сендай, способны вызывать мукозальный иммунитет и предотвращать вирусную инфекцию в слизистой респираторного тракта ‒ “входных воротах” патогена. Таким образом, встраивая консервативные антигенные детерминанты SARS-CoV-2 в геном вируса Сендай, можно создать вакцинную конструкцию, которая обеспечит эффективную и долговременную защиту от COVID-19.
Работа поддержана Программой фундаментальных исследований государственных академий наук на 2013‒2020 годы (тема № 01201363818), Государственным контрактом ФБУН ГНЦ ВБ “Вектор” Роспотребнадзора и РФФИ в рамках научного проекта № 18-34-00286.
Настоящая статья не содержит каких-либо исследований с участием людей или животных в качестве объектов исследований.
Авторы заявляют об отсутствии конфликта интересов.
Список литературы
(2020) COVID-19 vaccine development pipeline. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/.
Amanat F., Krammer F. (2020) SARS-CoV-2 vaccines: status report. Immunity. 52, 583‒589.
Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. (2020) Progress and prospects on vaccine development against SARS-CoV-2. Vaccines (Basel). 8, E153.
Chen W.H., Strych U., Hotez P.J., Bottazzi M.E. (2020) The SARS-CoV-2 vaccine pipeline: an overview. Curr. Trop. Med. Rep. 7, 61‒64.
Callaway E. (2020) The race for coronavirus vaccines: a graphical guide. Nature. 580, 576‒577.
(2017) Sanofi restricts dengue vaccine but downplays antibody enhancement. http://www.cidrap.umn.edu/ news-perspective/2017/12/sanofi-restricts-dengue-vaccine-downplays-antibody-enhancement.
Миронов А.Н., Супотницкий М.В., Лебединская Е.В. (2013) Феномен антитело-зависимого усиления инфекции у вакцинированных и переболевших. БИОпрепараты. Профилактика, диаг-ностика, лечение. 3, 12‒25.
Tirado S.M., Yoon K.J. (2003) Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 16, 69‒86.
Супотницкий М.В. (2016) Неугодная иммунология. Актуальная инфектология. 2, 73‒97.
Супотницкий М.В. (2016) Слепые пятна вакцинологии. Ред. Нестенко И.А., Москва: Русская панорама.
Smatti M.K., Al Thani A.A., Yassine H.M. (2018) Viral-induced enhanced disease illness. Front. Microbiol. 9, 2991.
Jaume M., Yip M.S., Kam Y.W., Cheung C.Y., Kien F., Roberts A., Li P.H., Dutry I., Escriou N., Daeron M., Bruzzone R., Subbarao K., Peiris J.S., Nal B., Altmeyer R. (2012) SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med. J. 18 (Suppl. 2), 31‒36.
Yip M.S., Leung N.H., Cheung C.Y., Li P.H., Lee H.H., Daeron M., Peiris J.S., Bruzzone R., Jaume M. (2014) Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 11, 82.
Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T., Zhu H., Liu J., Xu Y., Xie J., Morioka H., Sakaguchi N., Qin C., Liu G. (2016) Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect. Dis. 2, 361‒376.
Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daeron M., Bruzzone R., Peiris J.S., Jaume M. (2016) Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med. J. 22, 25‒31.
Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., Zhou Y., Du L., Li F. (2020) Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 94, e02015‒e02019.
Li L., Wo J., Shao J., Zhu H., Wu N., Li M., Yao H., Hu M., Dennin R.H. (2003) SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J. Clin. Virol. 28, 239‒244.
Yilla M., Harcourt B.H., Hickman C.J., McGrew M., Tamin A., Goldsmith C.S., Bellini W.J., Anderson L.J. (2005) SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 107, 93‒101.
Iwasaki A., Yang Y. (2020) The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 20, 339–341.
Jaume M., Yip M.S., Cheung C.Y., Leung H.L., Li P.H., Kien F., Dutry I., Callendret B., Escriou N., Altmeyer R., Nal B., Daeron M., Bruzzone R., Peiris J.S. (2011) Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 85, 10582‒10597.
Kam Y.W., Kien F., Roberts A., Cheung Y.C., Lamirande E.W., Vogel L., Chu S.L., Tse J., Guarner J., Zaki S.R., Subbarao K., Peiris M., Nal B., Altmeyer R. (2007) Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcγRII-dependent entry into B cells in vitro. Vaccine. 25, 729‒740.
Banerjee A., Nasir J.A., Budylowski P., Yip L., Aftanas P., Christie N., Ghalami A., Baid K., Raphenya A.R., Hirota J.A., Miller M.S., McGeer A.J., Ostrowski M., Kozak R.A., McArthur A.G., Mossman K., Mubareka S. (2020) Isolation, sequence, infectivity, and replication kinetics of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 26, 2054‒2063.
Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367, 1260‒1263.
Hoffmann M., Kleine-Weber H., Pohlmann S. (2020) A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 78, 779‒784.
(2020) RCSB PDP. https://www.rcsb.org/pdb/static.do?p=general_information/about_pdb/index.html#:~:text=The%20Protein%20Data%20Bank%20 (PDB,%2C%20other%20animals%2C%20and%20humans.
Ricke D., Malone R. (2020) Medical countermeasures analysis of 2019-nCoV and vaccine risks for antibody-dependent enhancement (ADE). Preprints. 2020030138. https://doi.org/10.20944/preprints202003.0138.v1
Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 181, 281‒292.
Yao H., Lu X., Chen Q., Xu K., Chen Y., Cheng L., Liu F., Wu Z., Wu H., Jin C., Zheng M., Wu N., Jiang C., Li L. (2020) Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. 2020.2004.2014.20060160. https://doi.org/10.1101/2020.04.14.20060160
Yuan M., Wu N.C., Zhu X., Lee C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. (2020) A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 368, 630‒633.
To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., Lau D.P., Choi C.Y., Chen L.L., Chan W.M., Chan K.H., Ip J.D., Ng A.C., Poon R.W., Luo C.T., Cheng V.C., Chan J.F., Hung I.F., Chen Z., Chen H., Yuen K.Y. (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 20, 565‒574. https://doi.org/10.1016/S1473-3099(20)30196-1
Lu L., Manopo I., Leung B.P., Chng H.H., Ling A.E., Chee L.L., Ooi E.E., Chan S.W., Kwang J. (2004) Immunological characterization of the spike protein of the severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 42, 1570‒1576.
Korber B., Fischer W., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Foley B., Giorgi E., Bhattacharya T., Parker M., Partridge D., Evans C., de Silva T., LaBranche C., Montefiori D. (2020) Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv. https://doi.org/10.1101/2020.04.29.069054
(2020) Mutation sites in the spike (S) protein. China National Center for Bioinformation. https://bigd.big.ac.cn/ ncov/protein?lang=en&fbclid=IwAR21Gq8ZjlozW1 TCfkXHYv8f4-7uM56GnzVOABYPzvZe3rd0gCDpyJeDPkA.
Adzhubei A.A., Eisenmenger F., Tumanyan V.G., Zinke M., Brodzinski S., Esipova N.G. (1987) Third type of secondary structure: noncooperative mobile conformation. Protein Data Bank analysis. Biochem. Biophys. Res. Commun. 146, 934‒938.
Adzhubei A.A., Sternberg M.J. (1993) Left-handed polyproline II helices commonly occur in globular proteins. J. Mol. Biol. 229, 472‒493.
Нечипуренко Ю.Д., Анашкина А.А., Матвеева О.В. (2020) Изменение антигенных детерминант S-белка вируса SARS-CoV-2 как возможная причина антителозависимого усиления инфекции и цитокинового шторма. Биофизика. 65, 824‒832.
Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. (2020) Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 369(6501), 330–333. https://doi.org/10.1126/science.abb9983
Lu I.N., Muller C.P., He F.Q. (2020) Applying next-generation sequencing to unravel the mutational landscape in viral quasispecies: a mini-review. Virus Res. 283, 197963.
Takada A., Kawaoka Y. (2003) Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13, 387‒398.
Perlman S., Dandekar A.A. (2005) Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 5, 917‒927.
Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z., Wu T., Che-ung K.W., Chan K.H., Alvarez X., Qin C., Lackner A., Perlman S., Yuen K.Y., Chen Z. (2019) Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 4, e123158.
Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. (2012) Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 7, e35421.
Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., Couch R.B., Tseng C.T. (2016) Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin Immunother. 12, 2351‒2356.
Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. (2011) A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 85, 12201‒12215.
Roberts A., Lamirande E.W., Vogel L., Baras B., Goossens G., Knott I., Chen J., Ward J.M., Vassilev V., Subbarao K. (2010) Immunogenicity and protective efficacy in mice and hamsters of a beta-propiolactone inactivated whole virus SARS-CoV vaccine. Viral. Immunol. 23, 509‒519.
Quinlan B.D., Mou H., Zhang L., Guo Y., He W., Ojha A., Parcells M.S., Luo G., Li W., Zhong G., Choe H., Farzan M. (2020) The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. bioRxiv. https://doi.org/10.1101/2020.04.10.036418
Yuan F.F., Tanner J., Chan P.K., Biffin S., Dyer W.B., Geczy A.F., Tang J.W., Hui D.S., Sung J.J., Sullivan J.S. (2005) Influence of FcγRIIA and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens. 66, 291‒296. https://doi.org/10.1111/j.1399-0039.2005.00476.x
Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hagglof T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H.H., et al. (2020) Convergent antibody responses to SARS-CoV-2 infection in convalescent individuals. Nature. 584, 437–442. https://doi.org/10.1038/s41586-020-2456-9
Zhang L., Zhang F., Yu W., He T., Yu J., Yi C.E., Ba L., Li W., Farzan M., Chen Z., Yuen K.Y., Ho D. (2006) Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J. Med. Virol. 78(1), 1‒8.
Tetro J.A. (2020) Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 22, 72‒73. https://doi.org/10.1016/j.micinf.2020.02.006
Супотницкий М.В. (2014) Феномен антигенного импринтинга в инфекционных, эпидемических и поствакцинальных процессах. БИОпрепараты. Профилактика, диагностика, лечение. 3, 27‒39.
Monto A.S., Malosh R.E., Petrie J.G., Martin E.T. (2017) The doctrine of original antigenic sin: separating good from evil. J. Infect. Dis. 215, 1782‒1788.
Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., Hu J.-L., Xu W., Zhang Y., Lv F.-J., Su K., Zhang F., Gong J., Wu B., Liu X.-M., Li J.-J., Qiu J.-F., Chen J., Huang A.-L. (2020) Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. https://doi.org/10.1038/s41591-020-0965-6
Jiang H.-w., Li Y., Zhang H.-n., Wang W., Men D., Yang X., Qi H., Zhou J., Tao S.-c. (2020) Global profiling of SARS-CoV-2 specific IgG/ IgM responses of convalescents using a proteome microarray. medRxiv. https://doi.org/10.1101/2020.03.20.20039495
Shang J., Wan Y., Liu C., Yount B., Gully K., Yang Y., Auerbach A., Peng G., Baric R., Li F. (2020) Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 16, e1008392.
Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., Ling Y., Zhang Y., Xun J., Lu L., Jiang S., Lu H., Wen Y., Huang J. (2020) Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. https://doi.org/10.1101/2020.03.30.20047365
Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., Baysal E., Mangold M., Henze L., Lauster R., Mall M., Beyer K., Roehmel J., Schmitz J., Miltenyi S., Mueller M.A., Witzenrath M., Suttorp N., Kern F., Reimer U., Wenschuh H., Drosten C., Corman V.M., Giesecke-Thiel C., Sander L.-E., Thiel A. (2020) Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. medRxiv. https://doi.org/10.1101/2020.04.17.20061440
Grifoni A., Weiskopf D., Ramirez S.I, Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. (2020) Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 181(7), 1489–1501.e15. https://doi.org/10.1016/j.cell.2020.05.015
Ahmed S.F., Quadeer A.A., McKay M.R. (2020) Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 12, 254.
Qamar M.T., Rehman A., Ashfaq U.A., Awan M.Q., Fatima I., Shahid F., Chen L.-L. (2020) Designing of a next generation multiepitope based vaccine (MEV) against SARS-COV-2: immunoinformatics and in silico approaches. bioRxiv. https://doi.org/10.1101/2020.02.28.970343
Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. (2020) SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39(10), e105114. https://doi.org/10.15252/embj.20105114
Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A., Schipper D., van Run P., Leijten L., Sikkema R., Verschoor E., Verstrepen B., Bogers W., Langermans J., Drosten C., Fentener van Vlissingen M., Fouchier R., de Swart R., Koopmans M., Haagmans B.L. (2020) Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 368, 1012‒1015.
Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I., Miao V.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth II M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Tzouanas C.N., Taliaferro F., Guo Z., Wang J.P., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Berger B.A., Leslie A., Flynn J.L., Fortune S.M., Finberg R.W., Kean L.S., Garber M., Schmidt A., Lingwood D., Shalek A.K., Ordovas-Montanes J., HCA Lung Biological Network. (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 181, 1016‒1035.
Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 30, 343‒355.
Gu J., Han B., Wang J. (2020) COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 158, 1518‒1519.
Lamm M.E. (1998) Current concepts in mucosal immunity. IV. How epithelial transport of IgA antibodies relates to host defense. Am. J. Physiol. 274, G614‒G617. https://doi.org/10.1152/ajpgi.1998.274.4.g614
Nyombayire J., Anzala O., Gazzard B., Karita E., Bergin P., Hayes P., Kopycinski J., Omosa-Manyonyi G., Jackson A., Bizimana J., Farah B., Sayeed E., Parks C.L., Inoue M., Hironaka T., Hara H., Shu T., Matano T., Dally L., Barin B., Park H., Gilmour J., Lombardo A., Excler J.L., Fast P., Laufer D.S., Cox J.H., Team S.S. (2017) First-in-human evaluation of the safety and immunogenicity of an intranasally administered replication-competent Sendai virus-vectored HIV type 1 Gag vaccine: induction of potent T-cell or antibody responses in prime-boost regimens. J. Infect. Dis. 215, 95‒104.
Rollier C.S., Reyes-Sandoval A., Cottingham M.G., Ewer K., Hill A.V. (2011) Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 23, 377‒382.
Ewer K.J., Lambe T., Rollier C.S., Spencer A.J., Hill A.V., Dorrell L. (2016) Viral vectors as vaccine platforms: from immunogenicity to impact. Curr. Opin. Immunol. 41, 47‒54.
van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E.R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Bissett C., Gilbride C., Williamson B.N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P.W., Scott D., Saturday G., de Wit E., Gilbert S.C., Munster V.J. (2020) ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. https://doi.org/10.1101/2020.05.13.093195
Bradley R.R., Lynch D.M., Iampietro M.J., Borducchi E.N., Barouch D.H. (2012) Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J. Virol. 86, 625‒629.
(2020) DRAFT landscape of COVID-19 candidate vaccines. https://www.who.int/blueprint/priority-diseases/ key-action/novel-coronavirus-landscape-ncov.pdf?ua=1.
(2020) Вакцину от коронавируса в России сделают из вируса кори (The coronavirus vaccine in Russia will be created using measles virus.). https://www.mk.ru/ science/2020/04/13/vakcinu-ot-koronavirusa-v-rossii-delayut-iz-vakciny-ot-kori.html.
Malczyk A.H., Kupke A., Prufer S., Scheuplein V.A., Hutzler S., Kreuz D., Beissert T., Bauer S., Hubich-Rau S., Tondera C., Eldin H.S., Schmidt J., Vergara-Alert J., Suzer Y., Seifried J., Hanschmann K.M., Kalinke U., Herold S., Sahin U., Cichutek K., Waibler Z., Eickmann M., Becker S., Muhlebach M.D. (2015) A highly immunogenic and protective Middle East respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J. Virol. 89, 11654‒11667. https://doi.org/10.1128/JVI.01815-15
Li K., Li Z., Wohlford-Lenane C., Meyerholz D.K., Channappanavar R., An D., Perlman S., McCray P.B., Jr., He B. (2020) Single-dose, intranasal immunization with recombinant parainfluenza virus 5 expressing middle east respiratory syndrome coronavirus (MERS-CoV) spike protein protects mice from fatal MERS-CoV infection. mBio. 11, e00554-20.https://doi.org/10.1128/mBio.00554-20
Lin G.Y., Lamb R.A. (2000) The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 74, 9152‒9166.
Yoshida A., Kim S.H., Manoharan V.K., Varghese B.P., Paldurai A., Samal S.K. (2019) Novel avian paramyxovirus-based vaccine vectors expressing the Ebola virus glycoprotein elicit mucosal and humoral immune responses in guinea pigs. Sci. Rep. 9, 5520.
Mohamed Amin Z., Che Ani M.A., Tan S.W., Yeap S.K., Alitheen N.B., Syed Najmuddin S.U.F., Kalyanasundram J., Chan S.C., Veerakumarasivam A., Chia S.L., Yusoff K. (2019) Evaluation of a recombinant Newcastle disease virus expressing human IL12 against human breast cancer. Sci. Rep. 9, 13999. https://doi.org/10.1038/s41598-019-50222-z
Vijayakumar G., McCroskery S., Palese P. (2020) Engineering Newcastle disease virus as an oncolytic vector for intratumoral delivery of immune checkpoint inhibitors and immunocytokines. J. Virol. 94, e01677-19.https://doi.org/10.1128/JVI.01677-19
(2020) Immunity and safety of Covid-19 synthetic minigene vaccine. https://clinicaltrials.gov/ct2/show/NCT04276896
Villenave R., Touzelet O., Thavagnanam S., Sarlang S., Parker J., Skibinski G., Heaney L.G., McKaigue J.P., Coyle P.V., Shields M.D., Power U.F. (2010) Cytopathogenesis of Sendai virus in well-differentiated primary pediatric bronchial epithelial cells. J. Virol. 84, 11718‒11728.
Luber C.A., Cox J., Lauterbach H., Fancke B., Selbach M., Tschopp J., Akira S., Wiegand M., Hochrein H., O’Keeffe M., Mann M. (2010) Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 32, 279‒289.
Skiadopoulos M.H., Surman S.R., Riggs J.M., Elkins W.R., St Claire M., Nishio M., Garcin D., Kolakofsky D., Collins P.L., Murphy B.R. (2002) Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology. 297, 153‒160.
Матвеева О.В., Кочнева Г.В., Нетесов С.В., Оникиенко С.Б., Чумаков П.М. (2015) Механизмы онколитического действия парамиксовируса Сендай. Acta Naturae. 7(2), 6‒16.
Slobod K.S., Shenep J.L., Lujan-Zilbermann J., Allison K., Brown B., Scroggs R.A., Portner A., Coleclough C., Hurwitz J.L. (2004) Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 22, 3182‒3186.
Adderson E., Branum K., Sealy R.E., Jones B.G., Surman S.L., Penkert R., Freiden P., Slobod K.S., Gaur A.H., Hayden R.T., Allison K., Howlett N., Utech J., Allay J., Knight J., Sleep S., Meagher M.M., Russell C.J., Portner A., Hurwitz J.L. (2015) Safety and immunogenicity of an intranasal Sendai virus-based human parainfluenza virus type 1 vaccine in 3- to 6-year-old children. Clin. Vaccine Immunol. 22(3), 298‒303. https://doi.org/10.1128/CVI.00618-14
Moriya C., Horiba S., Kurihara K., Kamada T., Takahara Y., Inoue M., Iida A., Hara H., Shu T., Hasegawa M., Matano T. (2011) Intranasal Sendai viral vector vaccination is more immunogenic than intramuscular under pre-existing anti-vector antibodies. Vaccine. 29, 8557‒8563.
Hara H., Hara H., Hironaka T., Inoue M., Iida A., Shu T., Hasegawa M., Nagai Y., Falsey A.R., Kamali A., Anzala O., Sanders E.J., Karita E., Mwananyanda L., Vasan S., Lombardo A., Parks C.L., Sayeed E., Krebs M., Cormier E., Ackland J., Price M.A., Excler J.L. (2011) Prevalence of specific neutralizing antibodies against Sendai virus in populations from different geographic areas: implications for AIDS vaccine development using Sendai virus vectors. Hum. Vaccin. 7, 639‒645.
Moriya C., Horiba S., Inoue M., Iida A., Hara H., Shu T., Hasegawa M., Matano T. (2008) Antigen-specific T-cell induction by vaccination with a recombinant Sendai virus vector even in the presence of vector-specific neutralizing antibodies in rhesus macaques. Biochem. Biophys. Res. Commun. 371, 850‒854.
Seki S., Matano T. (2016) Development of a Sendai virus vector-based AIDS vaccine inducing T cell responses. Expert Rev. Vaccines. 15, 119‒127.
Hu Z., Gu L., Li C.L., Shu T., Lowrie D.B., Fan X.Y. (2018) The profile of T cell responses in Bacille Calmette-Guerin-primed mice boosted by a novel Sendai virus vectored anti-tuberculosis vaccine. Front. Immunol. 9, 1796.
Hu Z., Jiang W., Gu L., Qiao D., Shu T., Lowrie D.B., Lu S.H., Fan X.Y. (2019) Heterologous prime-boost vaccination against tuberculosis with recombinant Sendai virus and DNA vaccines. J. Mol. Med. (Berl.). 97, 1685‒1694.
Russell C.J., Hurwitz J.L. (2016) Sendai virus as a backbone for vaccines against RSV and other human paramyxoviruses. Expert Rev. Vaccines. 15, 189‒200.
Wiegand M.A., Gori-Savellini G., Gandolfo C., Papa G., Kaufmann C., Felder E., Ginori A., Disanto M.G., Spina D., Cusi M.G. (2017) A respiratory syncytial virus vaccine vectored by a stable chimeric and replication-deficient Sendai virus protects mice without inducing enhanced disease. J. Virol. 91, e02298-16.https://doi.org/10.1128/JVI.02298-16
Han G.Z., Worobey M. (2011) Homologous recombination in negative sense RNA viruses. Viruses. 3, 1358‒1373.
Kolakofsky D., Roux L., Garcin D., Ruigrok R.W. (2005) Paramyxovirus mRNA editing, the “rule of six” and error catastrophe: a hypothesis. J. Gen. Virol. 86, 1869‒1877.
Kolakofsky D. (2016) Paramyxovirus RNA synthesis, mRNA editing, and genome hexamer phase: a review. Virology. 498, 94‒98.
Matsumoto Y., Ohta K., Kolakofsky D., Nishio M. (2018) The control of paramyxovirus genome hexamer length and mRNA editing. RNA. 24, 461‒467.
The Nextstrain team. (2020) Genomic epidemiology of novel coronavirus ‒ Global subsampling. 3246 genomes sampled between December 2019 and June 2020. https://nextstrain.org/ncov/global.
Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. (2020) Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. ciaa344. https://doi.org/10.1093/cid/ciaa344
Nakanishi M., Otsu M. (2012) Development of Sendai virus vectors and their potential applications in gene therapy and regenerative medicine. Curr. Gene Ther. 12, 410‒416.
Burke C.W., Mason J.N., Surman S.L., Jones B.G., Dalloneau E., Hurwitz J.L., Russell C.J. (2011) Illumination of parainfluenza virus infection and transmission in living animals reveals a tissue-specific dichotomy. PLoS Pathog. 7, e1002134.
Sakai Y., Kiyotani K., Fukumura M., Asakawa M., Kato A., Shioda T., Yoshida T., Tanaka A., Hasegawa M., Nagai Y. (1999) Accommodation of foreign genes into the Sendai virus genome: sizes of inserted genes and viral replication. FEBS Lett. 456, 221‒226.
Faisca P., Desmecht D. (2007) Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res. Vet. Sci. 82, 115‒125.
Hasan M.K., Kato A., Shioda T., Sakai Y., Yu D., Nagai Y. (1997) Creation of an infectious recombinant Sendai virus expressing the firefly luciferase gene from the 3' proximal first locus. J. Gen. Virol. 78(Pt 11), 2813‒2820.
Hurwitz J.L., Takimoto T., Russell C.J., Portner A., Slobod K. (2018) Modified Sendai virus vaccine and imaging vector. Patent US9637758B2.
Zhan X., Slobod K.S., Jones B.G., Sealy R.E., Takimoto T., Boyd K., Surman S., Russell C.J., Portner A., Hurwitz J.L. (2015) Sendai virus recombinant vaccine expressing a secreted, unconstrained respiratory syncytial virus fusion protein protects against RSV in cotton rats. Int. Immunol. 27, 229‒236.
Bitzer M., Armeanu S., Lauer U.M., Neubert W.J. (2003) Sendai virus vectors as an emerging negative-strand RNA viral vector system. J. Gene Med. 5, 543‒553.
Tatsumoto N., Arditi M., Yamashita M. (2018) Sendai virus propagation using chicken eggs. Bio Protoc. 8(18), e3009. https://doi.org/10.21769/BioProtoc.3009
Itoh M., Wang X.L., Suzuki Y., Homma M. (1992) Mutation of the HANA protein of Sendai virus by passage in eggs. Virology. 190, 356‒364.
Zainutdinov S.S., Grazhdantseva A.A., Kochetkov D.V., Chumakov P.M., Netesov S.V., Matveeva O.V., Kochneva G.V. (2017) Change in oncolytic activity of Sendai virus during adaptation to cell cultures. Mol. Genet., Mikrobiol. Virusol. 32(4), 156‒160. https://doi.org/10.3103/S0891416817040115
Zainutdinov S.S., Kochneva G.V., Netesov S.V., Chumakov P.M., Matveeva O.V. (2019) Directed evolution as a tool for the selection of oncolytic RNA viruses with desired phenotypes. Oncolytic Virother. 8, 9‒26. https://doi.org/10.2147/OV.S176523
Simon A.Y., Moritoh K., Torigoe D., Asano A., Sasaki N., Agui T. (2009) Multigenic control of resistance to Sendai virus infection in mice. Infect. Genet. Evol. 9, 1253‒1259.
Stone A.E., Giguere S., Castleman W.L. (2003) IL-12 reduces the severity of Sendai virus-induced bronchiolar inflammation and remodeling. Cytokine. 24, 103‒113.
Gretebeck L.M., Subbarao K. (2015) Animal models for SARS and MERS coronaviruses. Curr. Opin. Virol. 13, 123‒129.
Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Ma-rasco W.A. (2005) Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 79, 5900‒5906.
Carey K. (2020) Increasing number of biopharma drugs target COVID-19 as virus spreads. BioWorld. https://www.bioworld.com/articles/433331-increasing-number-of-biopharma-drugs-target-covid-19-as-virus-spreads
Zainutdinov S.S., Tikunov A.Y., Matveeva O.V., Netesov S.V., Kochneva G.V. (2016) Complete genome sequence of the oncolytic Sendai virus strain Moscow. Genome Announc. 4, e00818-16. https://doi.org/10.1128/genomeA.00818-16
Дополнительные материалы отсутствуют.
Инструменты
Молекулярная биология


