Российский физиологический журнал им. И.М. Сеченова, 2021, T. 107, № 2, стр. 221-231
Age-related changes of voluntary attention process
A. A. Tumanian1 1, *, N. E. Tadevosyan 1, A. S. Khachunts 1, I. G. Tadevosyan 1, E. G. Kostanyan 1, Z. H. Haji Beygi 1
1 Orbeli Institute of Physiology NAS RA
Yerevan, Armenia
* E-mail: tumanyanaa@mail.ru
Поступила в редакцию 23.06.2020
После доработки 17.11.2020
Принята к публикации 16.12.2020
Аннотация
The article covers the study of voluntary attention process considering age-related features of the subjects. In many studies, cognitive functions were mostly studied in two age groups – in the younger and the older subjects. In this study, changes in the voluntary attention process were studied in a wide age range (18–65 years), making it possible to trace their dynamics. The characteristics of voluntary attention were assessed using the “Clocks Carrousel” psychophysiological test, developed by analogy with the d2 Test in four age groups (the 1st group – 18–21 years old, the 2nd group – 22–35 years old, the 3rd group – 36–55 years old, the 4th group – 56–65 years old). The psychological state of all participants was assessed as stable based on results in Taylor Manifest Anxiety Scale and Pichot Inventory tests. The study of physiological state, by estimating heart rate (HR) and blood pressure (BP), showed that HR and BP were in age norm within all age groups. According to the psychophysiological study results, the highest level of voluntary attention, i.e., high speed of information processing, high concentration, and attention productivity, was found in the 1st group. The 1st group, in comparison with the other groups, spent less time on processing each symbol. A decrease in the level of attention was observed in the 2nd group. In the 3rd group, a tendency to its further decline was indicated. However, a more expressed decrease in the attention level was revealed in the 4th group compared to the 1st and 2nd groups. It should be noted that no significant differences were shown in the parameters of voluntary attention between the subjects of the 3rd and the 4th groups. We suppose that the revealed age-related changes in voluntary attention are due to the perception process impairment and a general slowing of information processing. At the same time, the older groups' subjects probably possess some degree of “latent reserve” that can be activated by spending additional time and energy.
Cognitive functioning refers to multiple mental abilities, including learning, thinking, reasoning, remembering, problem-solving, decision making, and attention [1]. Cognitive decline with aging can be explained by the Inhibitory Deficit Hypothesis, according to which older adults are more susceptible to irrelevant stimuli and face more difficulties in inhibiting distractions as compared to the younger population, resulting in heightened distractibility, poorer retrieval of task-relevant details, and overall worsened task performance [2].
Attention and speed of information processing are involved in virtually all cognitive domains. These functions are essential for the effective performance of higher-level everyday cognitive tasks such as decision-making, problem-solving, and the planning of goal-directed behaviors [3]. Visual attention and information processing are particularly relevant to the study of cognitive decline as most human behavior is visually driven. Studies have shown that the older adults are slower and less accurate on visual tasks performance (e.g., efficient visual-search tasks) reliant on bottom-up control of attention, i.e., inhibition of distractors and are driven by salient differences among the features of the stimuli, even when tested under best-corrected conditions (i.e., with glasses) [2]. Cognitive-aging research has also reported an age-related decline in top-down control of attention relating to the idea of age-related decline in executive processing. Executive function is a key component of attention and is defined as processes involved in maintaining and updating information in working memory. The impairment of executive function is one of the key contributors to age-related declines in a range of cognitive tasks [3, 4]. Older adults' decreased performance in visual-search tasks is not entirely due to bottom-up processing and includes some decline in top-down attentional control [4].
On the other hand, when some visual tasks (e.g., inefficient visual-search tasks) require the different forms of top-down control of attention, which are facilitated by advanced knowledge of the target-defining feature, individual’s internal valuations, goals, or perceptual-sets, older adults perform as fast and as accurately as younger adults [2]. Older adults often have less activity in the occipital lobe mediating the visual processing, reflecting an age-related decline in the bottom-up sensory input quality. In response to altered function in some brain regions, older adults typically overrecruit other brain areas, mainly the frontal and parietal areas, during tasks engaging cognitive control processes, such as attention. Increased frontoparietal activation represents an increase in the role of top-down attentional control. This over-recruitment may be a compensatory response to a decline in the bottom-up input from sensory pathways in the older adults [4, 5]. Normally aging older adults often utilize different brain areas and functional brain networks than young people do when performing the same cognitive tasks. In some cases, this differential brain activity in older adults has been accompanied by equivalent cognitive performance to that seen in the young subjects, suggesting that greater reliance on this brain region is related in some way to the maintained ability of the older individuals to perform the task [6].
The study of cognitive processes, in particular attentional processes, depending on age-related changes is essential for assessing the general psychophysiological state and older adult’s ability to function efficiently [7]. Works are known, where a comparative study of cognitive processes was carried out only in the young and the older groups of subjects, which did not give an idea of the age dynamics of cognitive functions [2, 8–11].
Thus in this study, age-related changes in cognitive processes were considered in four age groups of subjects (age from 18 to 65 years old), which made it possible to trace their dynamics. The study of the voluntary attention process comparing quantitative, temporal, and qualitative characteristics of attention made it possible to get a more precise and more complete idea of age-related voluntary attention changes.
Thus the current study aimed at comparing the quantitative, temporal, and qualitative characteristics of voluntary attention in different age groups, using the computerized psychophysiological test while assessing psychological state.
METHODS
Participants
We studied 72 healthy volunteers divided into four age groups according to the Scheme of age periodization of human ontogenesis (adopted at the 7th All-Union Conference on the Problems of Age Morphology, Physiology, and Biochemistry, APS USSR, Moscow) [12]. The 1st group included 22 subjects [mean age ± SD (min-max): 19.7 ± 1.3 (18–21) years, 13 men and 9 women], the 2nd group included 20 subjects [25.7 ± 3.7 (22–35) years, 7 men and 13 women], the 3rd group included 17 subjects [46.9 ± 4.9 (36–55) years, 6 men and 11 women], the 4th group included 13 subjects [62.4 ± 2.8 (56–65) years, 7 men and 6 women]. All participants had normal or corrected to normal vision and were right-handed, with no history of neurological or psychiatric episodes. None of the subjects were on medication or suffered from chronic or acute disease. The study was operated in accordance with the World Medical Association’s Declaration of Helsinki. All participants provided written and informed consent before enrollment.
Psychological Test
The adapted psychological computerized tests (Taylor Manifest Anxiety Scale (TMAS) and Pichot Inventory) were used to study the psychological state [13].
Taylor Manifest Anxiety Scale (TMAS)
TMAS is a self-report measure designed to assess anxiety level and consists of 50 items, each requiring a “yes” or “no” answer. True-false responses are used for each item, and the replies indicating anxiety are counted, giving a score from 0 to 50, with higher scores representing a higher level of anxiety. It takes about 10–15 minutes to complete the test. Norms: 41–50 scores – very high level of anxiety, 26–40 scores – high level of anxiety, 16–25 scores – the average level of anxiety (with a tendency to high level), 6–15 scores – the average level of anxiety (with a tendency to low level), 0–5 scores – low level of anxiety [14].
Pichot Inventory
Pichot inventory is a self-rating inventory of 39 items that includes three subscales of 13 items each, measuring 3 aspects of mood (depression, asthenia, and hypochondria). True-false responses are used for each item, and the replies indicating depression, asthenia, and hypochondria are counted, giving a score from 0 to 100 with higher scores representing more negative mood. It takes about 10–15 minutes to complete the test. Norms: 71–100 scores – high level of depression, asthenia, and hypochondria, 31–70 scores – the average level of depression, asthenia, and hypochondria, 0–30 scores – low level of depression, asthenia, and hypochondria [15].
Psychophysiological Test
For the cognitive assessment, all individuals performed the psychophysiological test. The test was duly explained to the subjects, and sufficient trials were given for proper understanding. The computerized psychophysiological test using custom software “Clocks Carrousel” was used to study voluntary attention, in particular selective attention [16]. This test was developed by analogy with the d2 Test by incorporating some modifications [17]. In “Clocks Carrousel,” stimuli were presented by rings similar to the clock dial without numerals and with only hour hand (all hours besides 3, 6, 9, and 12) arranged in a circle in the center of the display (as opposed to characters arranged in lines in d2 Test). The display size was 19" (width × height։ 16.56" × 9.31"), the diameter of each stimulus was 2.2 cm, and the diameter of the circle was 19.3 cm. The sequence of stimuli changed automatically before 10% of the completion of the circle. Stimuli were divided into two groups – control symbols (clock dial with the hour hand pointing at 5 and 10) and ordinary symbols (clock dial with the hour hand pointing at 1, 2, 4, 7, 8, and 11) (See fig.1).
Fig. 1.
A – Screenshot of the working window of the psychophysiological test “Clocks Carrousel.” B – Control and ordinary symbols.
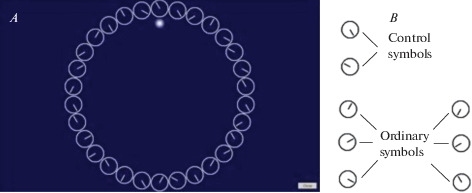
The subjects were at a distance of 60–70 cm from the display. The subjects were instructed to pass from symbol to symbol by pressing the cursor control key, thus fixing each symbol’s processing time and press the spacebar immediately when they saw the control symbol on the screen and omit the ordinary symbol while keeping the index finger on the spacebar. The fixed duration of the test was 10 minutes. All correctly clicked (choice of control symbols) and correctly missed symbols (omission of ordinary symbols) were considered as right responses. The incorrectly missed (omission of control symbols) and incorrectly clicked symbols (choice of ordinary symbols) were considered as errors.
The quantitative, qualitative, and temporal characteristics of voluntary attention were assessed. The quantitative parameters of the test are: TNS – total number of symbols (the number of symbols watched in ten minutes); NWS/min – number of watched symbols per minute; IMS – number of incorrectly missed symbols; ICS – number of incorrectly clicked symbols; TNE – total number of errors (ICS + IMS); TNRR – total number of right responses (TNS-TNE). In this article, the percentage of IMS, ICS, TNE, and TNRR parameters from TNS was calculated and studied (IMS%, ICS%, TNE%, and TNRR%, respectively). The qualitative parameters of the test are: CI – concentration index (CCS-ICS, where CCS is number of correctly clicked symbols); AP/min – attention productivity per minute (TNS*(CCS/(CCS + IMS)) [18, 19]. Time parameters were also studied: CCS_pt – correctly clicked symbols processing time (msec); CMS_pt – correctly missed symbols processing time (msec); IMS_pt – incorrectly missed symbols processing time (msec); ICS_pt – incorrectly clicked symbols processing time (msec).
Study Procedure
For comparability of the results, the study was carried out at the same time (at 11.00 h) in a quiet room. At first, all participants completed TMAS and Pichot inventory. Then they took the Psychophysiological test. Before starting the psychological and psychophysiological tests, all participants’ pulse and blood pressure (BP) were measured in the sitting position. The pulse was measured by palpation on A. radialis in one minute. BP was measured three times by Automatic Blood Pressure Monitor (Omron M6). The device also gave out the pulse value. The average value of three measurements of pulse and BP was calculated. Pulse results were generally comparable.
The psychological characteristics and HR and BP parameters were considered to assess the subjects’ general psychological and physiological states. Subjects with high severity of psychological characteristics and high values of pulse and BP (over the age norm) were excluded from the study.
Statistical analysis
Data are presented as mean ± SD. The normality of the data and the homogeneity of variance were determined by the Shapiro–Wilk test and the Levene’s test, respectively. Comparisons of age-dependent changes of characteristics of voluntary attention (total number of symbols, number of watched symbols per minute, number of incorrectly missed symbols, number of incorrectly clicked symbols, the total number of errors, total number of right responses, concentration index, attention productivity per minute, correctly clicked symbols processing time, correctly missed symbols processing time, incorrectly omitted symbols processing time, incorrectly clicked symbols processing time) were performed using the One-Way ANOVA for Gaussian distribution and the homogeneity of variance. When the ANOVA indicated significant age effects, significant differences between means were tested applying post hoc analysis with the Bonferroni correction. Kruskal–Wallis H-test was applied for Gaussian approximation, and Mann–Whitney posthoc test was used when necessary to evaluate significant interactions. A significant difference was indicated by p < 0.05. All statistical analysis was performed using the GraphPad Prism 5 software and SPSS (Version 16.0 for Windows).
RESULTS
Assessment of TMAS and Pichot inventory
The TMAS and Pichot inventory results are presented in Table 1.
Table 1.
Results of TMAS, Pichot inventory, pulse and blood pressure of the study groups
| Parameter | Group | |||
|---|---|---|---|---|
| 1st group (n = 22, 18–21 y.o.) |
2nd group (n = 20, 22–35 y.o.) |
3rd group (n = 17, 36–55 y.o.) |
4th group (n = 13, 56–65 y.o.) |
|
| Anxiety, c.u. | 17.8 ± 5.9 | 17.0 ± 7.7 | 20.2 ± 7.7 | 17.4 ± 6.0 |
| Depression, c.u. | 19.9 ± 8.7 | 15.0 ± 7.9 | 32.3 ± 12.2# | 34.0 ± 12.5*## |
| Asthenia, c.u. | 28.6 ± 10.5 | 17.6 ± 7.7 | 40.4 ± 14.3### | 43.9 ± 16.8## |
| Hypochondria, c.u. | 15.6 ± 6.8 | 12.1 ± 6.1 | 15.9 ± 8.8 | 13.1 ± 6.5 |
| HR, bpm | 71.6 ± 8.4 | 77.8 ± 7.6 | 80.5 ± 9.6* | 73.6 ± 9.9 |
| SBP, mm Hg | 110.2 ± 13.2 | 105.8 ± 11.9 | 105.1 ± 11.6 | 126.4 ± 10.4#Δ |
| DBP, mm Hg | 72.1 ± 10.0 | 66.8 ± 8.9 | 67.9 ± 11.4 | 83.0 ± 5.7#Δ |
Data are reported as Mean ± SD; * Significant difference in comparison with the 1st group; # Significant difference in comparison with the 2nd group; Δ Significant difference in comparison with the 3rd group; *p < 0.05, **p < 0.01, ***p < 0.001. HR: heart rate; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
There were no significant differences in the level of anxiety and hypochondria between the groups (F3, 68 = 0.68, p > 0.05 for anxiety and χ2 = 0.69, p > 0.05 for hypochondria). Most of the subjects in the groups had an average level of anxiety with tend to the high level and the low level of hypochondria. The Kruskal–Wallis H-test and ANOVA revealed significant age effect for depression and asthenia (χ2 = 9.38, p < 0.05 for depression and F3, 68 = 7.47, p < 0.001 for asthenia). Post-hoc tests showed that the level of depression and asthenia was significantly higher in the 3rd and the 4th groups than the 2nd group. The 4th group also had a higher level of depression than the 1st group. There were no other differences in the level of depression and asthenia between the groups. So the 1st and 2nd groups had low levels, and the 3rd and the 4th groups had an average level of depression and asthenia.
Assessment of pulse and blood pressure
Table 1 also shows the pulse and blood pressure parameters of subjects included in this study. The ANOVA indicated significant age effect for pulse and blood pressure (F3, 68 = 3.85, p < 0.05 for HR, F3, 68 = 1.92, p < 0.05 for SP and F3, 68 = 1.46, p < 0.05 for DP). Post-hoc analysis revealed that HR was significantly higher in the 3rd group compared to the 1st group. The SBP and DBP were significantly higher in the 4th group than in the 2nd and the 3rd groups. No other differences in HR, SBP and DBP were observed between the groups.
Psychophysiological study
The results of the psychophysiological study are summarized in Table 2.
Table 2.
Results of Psychophysiological test of the study groups
| Parameter | Group | |||
|---|---|---|---|---|
| 1st group (n = 22, 18–21 y.o.) |
2nd group (n = 20, 22–35 y.o.) |
3rd group (n = 17, 36–55 y.o.) |
4th group (n = 13, 56–65 y.o.) |
|
| TNS | 804.8 ± 76.5 | 665.0 ± 77.3*** | 622.6 ± 71.3*** | 592.1 ± 71.4***# |
| NWS/min | 81.4 ± 12.6 | 67.1 ± 12.0*** | 62.3 ± 9.1*** | 60.0 ± 7.6***# |
| TNRR, % | 94.1 ± 3.2 | 95.4 ± 3.8 | 96.2 ± 3.0 | 95.5 ± 2.2 |
| TNE, % | 6.0 ± 1.9 | 4.6 ± 1.8 | 3.9 ± 1.9* | 4.5 ± 1.4* |
| IMS, % | 4.8 ± 1.7 | 3.8 ± 1.8 | 2.9 ± 1.3* | 3.4 ± 1.9* |
| ICS, % | 1.2 ± 0.6 | 0.8 ± 0.5 | 1.1 ± 0.5 | 1.0 ± 0.4 |
| CI, c.u. | 272.8 ± 43.2 | 233.4 ± 39.7** | 224.5 ± 36.9** | 210.6 ± 30.2**# |
| AP/min, c.u. | 70.4 ± 10.6 | 59.7 ± 9.5** | 57.8 ± 9.0*** | 54.1 ± 6.9***# |
| CCS_pt, ms | 996.3 ± 149.7 | 1245.1 ± 234.7*** | 1325.4 ± 250.3*** | 1319.2 ± 138.6** |
| CMS_pt, ms | 324.3 ± 56.2 | 384.4 ± 65.0* | 400.4 ± 60.8** | 435.6 ± 90.5*** |
| ICS_pt, ms | 1190.0 ± 240.0 | 1647.1 ± 600.5** | 1517.7 ± 463.2** | 2002.0 ± 560.5***#Δ |
| IMS_pt, ms | 586.2 ± 123.8 | 727.6 ± 222.9* | 781.6 ± 161.2*** | 761.4 ± 157.3** |
Data are reported as Mean ± SD; * Significant difference in comparison with the 1st group; # Significant difference in comparison with the 2nd group; Δ Significant difference in comparison with the 3rd group; *p < 0.05, **p < 0.01, ***p < 0.001. TNS: total number of symbols; NWS/min: number of watched symbols per minute; TNRR: total number of right responses; TNE: total number of errors; IMS: number of incorrectly missed symbols; ICS: number of incorrectly clicked symbols; CI: concentration index; AP/min: productivity of attention per minute; CCS_pt: correctly clicked symbols processing time; CMS_pt: correctly missed symbols processing time; IMS_pt: incorrectly missed symbols processing time; ICS_pt: incorrectly clicked symbols processing time.
The ANOVA revealed a main effect of age for TNS and NWS/min (F3, 68 = 26.45, p < 0.001 for TNS and F3, 68 = 12.57, p < 0.001 for NWS/min). The TNS and NWS/min were significantly more in the 1st group compared to the other groups. The 4th group also had less TNS and NWS/min than the 2nd group. There were no other differences in these parameters between the groups. There were no effects of age for TNRR% (χ2 = 6.33, p > 0.05). A further analysis showed significant differences in the TNE% and IMS% between the study groups (F3, 68 = 4.59, p < 0.01 for TNE% and F3, 68 = 6.13, p < 0.001 for IMS%). Post-hoc analysis showed that the 1st group had significantly more TNE% and IMS% than the 3rd and 4th groups. No other differences in these parameters were found between the groups. There were no significant differences in the ICS% between the groups (χ2 = 3.50, p > 0.05). The ANOVA indicated significant age effect for CI and AP/min (F = 7.40, p < 0.001 for CI and F3, 68 = 8.83, p < 0.001 for AP/min). Table 2 shows that CI and AP/min were significantly more in the 1st group than in the other groups. The 2nd group also had more CI and AP/min compared with the 4th group. No other differences in CI and AP/min were revealed between groups.
The ANOVA and Kruskal–Wallis H-test revealed significant age effect for all time parameters (F3, 68 = 10.29, p < 0.001 for CCS_pt, F3, 68 = 7.69, p < 0.001 for CMS_pt, χ2 = 18.94, p < 0.001 for ICS_pt and χ2 = 17.20, p < 0.001 for IMS_pt). Post-hoc analysis of the processing time showed that all time parameters were markedly less in the 1st group than the other groups. The 4th group had significantly more ICS_pt than the 2nd and the 3rd groups. There were no other differences in time parameters between study groups. In all groups CCS_pt and CMS_pt were less than ICS_pt and IMS_pt, respectively.
DISCUSSION
The general psychological state assessment revealed no differences in the level of anxiety and hypochondria between the groups. Subjects of 36–65 years old had a higher level of depression and asthenia than younger subjects. Nevertheless, the severity of depression and asthenia in the 3rd and 4th groups was moderate. Our findings are inconsistent with those suggesting that older adult groups had lower levels of anxiety and depression than younger adults [20]. Personality factors are important determinants of cognitive functioning in older age. Neuroticism is one of the factors of personality structure and involves the manifestation of tension and experience of anxiety, anger, and depression. Neuroticism negatively affects cognitive functioning [21]. In our study, all subjects had low and moderate severity of psychological characteristics, i.e., the general psychological state of the subjects was assessed as a stable during the study.
The study of the physiological state by HR and BP parameters revealed a relatively high HR in the 3rd group and elevated BP in the 4th group. Despite some differences in HR and BP between groups, these parameters were within the age norm in all groups, according to [25]. It is known that resting heart rate is independently associated with cognitive decline and may be an important risk marker [22]. Studies of BP in the general population demonstrate associations between higher systolic BP and diastolic BP and cognitive impairment [23]. Midlife high BP is a well-known risk factor for cerebrovascular disease and, consequently, cognitive decline in old age. Observational studies indicate that a lower rather than a more elevated BP in old age increases the risk for cognitive decline. Lowering their BP may compromise cerebral blood flow and cognitive function [24].
It should be noted that in our study, no relationships were seen between the general psychological/physiological state and voluntary attention process in study groups. This is probably because the subjects had low and moderate severity of psychological characteristics, and HR and BP were within the age norm.
The study of the process of voluntary attention revealed some age-related changes. In the groups, the number of watched symbols, i.e., the amount of processed information and its processing speed, decreased with age, which was most expressed in the 4th group. There are data according to which processing speed peaks in the third decade of life and then begins to decline, which continues throughout the lifespan [26]. Another study has reported a continuous, regular decline in processing speed from an earlier age, beginning in the 20s, which was also observed in our work [20]. Despite the absence of differences in the number of right responses, i.e., general performance, between groups, the 3rd, and the 4th groups had fewer errors than the 1st group due to a decrease in the number of IMS. The analysis of the type of errors showed that the number of IMS in all study groups exceeded the number of ICS, regardless of age.
Significant changes in time parameters were revealed depending on age. In our study, the time for identification and differentiation of all correct and incorrect symbols increased with age, which was most expressed for ICS_pt. Age-related changes in time parameters, such as a significant increase in threshold exposure time to discriminate and identify simple visual stimuli in subjects of 60–81 age group compared with subjects of 18–29 age group, were identified by Ebaid and colleagues [2]. At the same time, there are no data on the age dynamics of time parameters. It should be noted that in our study, changes in time parameters were observed starting from 22 years of age. According to the time parameters, subjects of all groups required more time to differentiate incorrect symbols.
Analysis of quantitative parameters revealed some increase in performance accuracy in subjects aged from 36 to 65 years (the 3rd and 4th groups) compared with subjects aged from 18–21 years (the 1st group). These differences between groups may be due to higher impulsivity in subjects of the 1st group. The results of the study of Lufi and Colleagues indicated no differences on measures of impulsivity between the two age groups (31 and 75 years old) [8]. However, in this work, characteristics of attention were not studied in subjects under 31. Some decrease in the number of errors in the 3rd and 4th groups can also be explained by the fact that the subjects of these groups spent more time identifying and comparing each symbol and had less amount of processed information and lower processing speed compared to those of each symbol with the 1st group.
The age-related changes in quantitative and time parameters described above were also expressed in the qualitative parameters – CI and AP. A gradual decrease in concentration and productivity of attention was observed with age.
Thus, the current study demonstrates that voluntary attention is the highest in the 1st group. This group exceeded other groups in the amount and speed of processed information. Besides, the 1st group required less time to identify and differentiate symbols. The 1st group showed a high level of concentration and productivity of attention, which also pointed to a high level of voluntary attention. Changes in the voluntary attention process were started to be detected in the 2nd group. Although the accuracy of performance and general performance didn’t change in the 2nd group, the amount and speed of processed information significantly decreased, which was combined with an increase in the time for identification and differentiation. At the same time, the 2nd group showed a significant decrease in concentration and productivity of attention. In the 3rd group, a tendency to further decrease in the level of attention was observed. However, more expressed decline in attention level was revealed in the 4th group compared with the 1st and 2nd groups.
Interestingly, there were no significant differences in the study parameters between the 3rd and the 4th groups. The age-related impairment of voluntary attention is probably due to the perception process impairment and a general slowing of information processing. This viewpoint is also presented in the work of Glisky [3].
We suppose that the revealed age-related changes in the process of voluntary attention are a result of a decrease in functional network efficiency [27]. A reorganization of the functional and structural connectivity of the brain occurs with age. A decrease in connectivity within several cerebral networks, including the executive network, is associated with age-related cognitive decline [10]. However, age-related decline in attention is not entirely pervasive. Older adults possess some degree of “latent reserve” that can be activated by spending additional time and energy [20].
Список литературы
Fisher G.G., Chacon M., Chaffee D.S. Work Across the Lifespan. Chapter 2 – Theories of Cognitive Aging and Work. Eds. Baltes B.B., Rudolph C.W., Zacher H. Acad. Press. 17–45. 2019. https://doi.org/10.1016/B978-0-12-812756-8.00002-5
Ebaid D., Crewther S.G. Visual Information Processing in Young and Older Adults. Front. Aging Neurosci. 11: 116. 2019. https://doi.org/10.3389/fnagi.2019.00116
Glisky E.L. Brain Aging: Models, Methods, and Mechanisms. Chapter 1 - Changes in Cognitive Function in Human Aging. Eds. Riddle D.R. Boca Raton (FL): CRC Press/Taylor & Francis. 2007.
Madden D.J. Aging and Visual Attention. Curr. Dir. in Psychol. Sci. 16(2): 70–74. 2007. https://doi.org/10.1111/j.1467-8721.2007.00478.x
Grady C.L. Cognitive Neuroscience of Aging. Ann. NY Acad. Sci. 1124: 127–144. 2008. https://doi.org/10.1196/annals.1440.009
Grady C.L. Functional Brain Imaging and Age-Related Changes in Cognition. Biol. Psychol. 54(1–3): 259–281. 2000. https://doi.org/10.1016/s0301-0511(00)00059-4
Touroutoglou A., Zhang J., Andreano J.M., Dickerson B.C., Feldman Barrett L. Dissociable Effects of Aging on Salience Subnetwork Connectivity Mediate Age-Related Changes in Executive Function and Affect. Front. Aging Neurosci. 10: 410. 2018. https://doi.org/10.3389/fnagi.2018.00410
Lufi D., Segev S., Blum A., Rosen T., Haimov I. The Effect of Age on Attention Level: A Comparison of Two Age Groups. Int. J. Aging Hum. Dev. 81(3): 176–188. 2015. https://doi.org/10.1177/0091415015614953
Williams R.S., Biel A.L., Dyson B.J., Spaniol J. Age Differences in Gain- and Loss-Motivated Attention. Brain Cogn. 111: 171–181. 2017. https://doi.org/10.1016/j.bandc.2016.12.003
Ramanoël S., York E., Le Petit M., Lagrené K., Habas C., Arleo A. Age-Related Differences in Functional and Structural Connectivity in the Spatial Navigation Brain Network. Front. Neural Circuits. 13: 69. 2019. https://doi.org/10.3389/fncir.2019.00069
Folville A., Bahri M.A., Delhaye E., Salmon E., D’Argembeau A., Bastin C. Age-Related Differences in the Neural Correlates of Vivid Remembering. NeuroImage. 206: 116336. 2020. https://doi.org/10.1016/j.neuroimage.2019.116336
Алейникова Т.В. Возрастная психофизиология. Глава 1 – Представления о возрастной периодизации онтогенеза человека. Ростов-на Дону. УНИИ валеологии РГУ. 5–10. 2002. [Aleinikova T.V. Age-Specific Psychophysiology. Chapter 1 – Concepts of the Age Periodization of Human Otogenesis. Rostov-on-Don: ERI of Valueology of RSU. 5–10. 2002. (In Russ)].
Геворкян Э.Г., Оганесян Н.М. Оценка функционального состояния мозга с помощью компьютерных технологий диагностики. Мед. наука Армении. 46(1): 106–110. 2006. [Gevorkyan E.G., Oganesyan N.M. Assessment of the Brain Functional States with the Aid of Computer Diagnostic Technologies. Med. Sci. Armenia. 46(1): 106–110. 2006. (In Russ)].
Дерманова И.Б. Личностная шкала проявлений тревоги (Тейлор Дж., адаптация Немчина Т.А.). Диагностика эмоционально-нравственного развития. Ред. и сост. Дерманова И.Б. 26–128. 2002. [Dermanova I.B. Personal Scale of Manifestations of Anxiety (Taylor J., adaptation of Nemchin T.A.). Diagnostics of emotional-moral development. Ed. Dermanova I.B. St. Petersburg. Rech. 126–128. 2002. (In Russ)].
Нафтульева А.И. Психологическое тестирование: тесты (Пишо П., пер. с фр. Кружилина И.). Науч. ред. пер. на рус. яз. Нафтульева А.И. СПб. Питер. 2003. [Naftuleva A.I. Psychological Testing: tests (Pichot P., transl. from French Kruzhilina I.). Ed. Naftuleva A.I. SPb. Piter. 2003. (In Russ)].
Gevorkyan E.G. About Short-Term Fluctuations of Functional Activity of Human Brain. Materials of the Conference “The Modern Problems of Integrative Activity and Plasticity of the Nervous System”. 98–102. 2009.
Brickenkamp R. Test d2, Aufmerksamkeits-Belastungs-Test. Handanweisung. 8th expanded and revised edition. Göttingen. Germany. Hogrefe. 1994.
Brickenkamp R. d2, Test de Atención: Manual. Adaptación española: Nicolas Seisdedos Cubero. 4ed. Madrid: TEA Ediciones, S.A.U. 2012. I.S.B.N.:978-84-15262-68-8
Сидоров К.Р. Количественная оценка продуктивности внимания в методике “Корректурная проба” Б. Бурдона. Вестн. Удмуртск. универ. 4. 50–57. 2012. [Sidorov K.R. Qualitative Assessment of the Efficiency of Attention in the Methodology of “Cancellation Test” by B. Bourdon. Bull. Udmurt. State Univer. 4: 50–57. 2012. (In Russ)].
Anderson N.D., Craik F.I. 50 Years of Cognitive Aging Theory. J. Gerontol. B Psychol. Sci. Soc. Sci. 72(1): 1–6. 2017. https://doi.org/10.1093/geronb/gbw108
Arshad T., Kausar R., Fatima I. Psychosocial Determinates of Cognitive Functioning in Older Adults. J. Liaquat. Uni. Med. Health Sci. 19(03): 208–214. 2020. https://doi.org/10.22442/jlumhs.201930692
Leong D.P., O’Donnell M.J., Teo K., Smyth A., Joseph P., Gao P., Bohm M.J., Yusuf S. Resting Heart Rate and Decline in Cognitive Function: Observations from the ONTARGET/TRANSCEND Studies. Europ. Heart J. 34(suppl_1, P2737): 499–500. 2013. https://doi.org/10.1093/eurheartj/eht309.P2737
Drew D.A., Tighiouart H., Duncan S., Rollins J., Gupta A., Scott T., Weinea D.E., Sarnak M.J. Blood Pressure and Cognitive Decline in Prevalent Hemodialysis Patients. Am. J. Nephrol. 49: 460–469. 2019. https://doi.org/10.1159/000500041
Moonen J.E.F., Foster-Dingley J.C., de Ruijter W. Effect of Discontinuation of Antihypertensive Treatment in Elderly People on Cognitive Functioning—the DANTE Study Leiden: A Randomized Clinical Trial. JAMA Intern. Med. 175(10): 1622–1630. 2015. https://doi.org/10.1001/jamainternmed.2015.4103
Lin J.-D., Chen Y.-L., Wu C.-Z., Hsieh C.-H., Pei D., Liang Y.-J., Chang J.-B. Identification of Normal Blood Pressure in Different Age Group. Medicine (Baltimore). 95(14): e3188. 2016. https://doi.org/10.1097/MD.0000000000003188
Harada C.N., Natelson Love M.C., Triebel K.L. Normal cognitive aging. Clin. Geriatr. Med. 29(4): 737–752. 2013. https://doi.org/10.1016/j.cger.2013.07.002
Niu H., Zhu J., Wang C., Zhu L., Wu J. Changes in White-Matter Functional Network Efficiency Across the Adult Lifespan. Neuroreport. 30(8): 600–604. 2019. https://doi.org/10.1097/WNR.0000000000001255
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова


