Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 12, стр. 1763-1779
Свободный холин крови как биомаркер физиологического статуса организма
Е. И. Савельева 1, *, М. А. Ленинский 1, Н. В. Гончаров 1, 2
1 НИИ гигиены, профпатологии и экологии человека
г.п. Кузьмоловский, Ленинградская обл., Россия
2 Институт эволюционной физиологии и биохимии им. И.М. Сеченова РАН
Санкт-Петербург, Россия
* E-mail: saveleva@gpeh.ru
Поступила в редакцию 12.09.2023
После доработки 11.10.2023
Принята к публикации 16.10.2023
- EDN: CHVWDP
- DOI: 10.31857/S0869813923120099
Аннотация
Холин является важнейшим нутриентом. С недостаточным потреблением холина связывают митохондриальные дисфункции, оксидативный стресс и обусловленные этими процессами риски развития неалкогольной жировой болезни печени, сердечно-сосудистой патологии, мышечной дистрофии, патологии развития нервной трубки плода. При этом избыточное потребление холина связывают с накоплением в крови уремического токсина – триметиламиноксида, биопредшественником которого является не фосфатидилхолин, а свободный холин крови. Содержание холина и его метаболитов в плазме крови ассоциировано с разными типами сосудистых патологий, позволяет прогнозировать тяжесть течения сердечно-сосудистых и других ассоциированных с ними заболеваний. Противоречивые сведения о норме и отклонениях от нормы содержания свободного холина в плазме крови в числе прочих факторов обусловлены недостаточным вниманием к стабилизации содержания свободного холина в плазме крови на этапах, предшествующих инструментальному анализу. Использование ЭДТА в качестве антикоагулянта и соблюдение холодового режима (не выше 4°С) сразу же после отбора крови и до инструментального анализа позволяет избежать роста концентрации холина в плазме ex vivo.
ВВЕДЕНИЕ
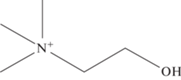
Холин ((2-гидроксиэтил)триметиламмоний) – витаминоподобное вещество, поступающее в организм с пищей, а также способное быть синтезированным de novo [1]. Структурная формула холина представлена на рис. 1.
Рис. 1.
Структурная формула холина. Брутто-формула: C5H14NO, молярная масса: 104,1708 г/моль, рег. номер CAS: 62-49-7, рег. номер EINECS: 200-535-1.
Холин вовлечен в реализацию ряда важнейших физиологических функций: является предшественником нейромедиатора ацетилхолина и агонистом альфа-7-никотиновых рецепторов; необходим для укрепления клеточных мембран; является донором метильных групп для синтеза S-аденозилметионина (SAM). Полагают, что холин и фолат, взаимодействуя с витамином B12, действуют как доноры метила для гомоцистеина с образованием метионина, который затем превращается в SAM, являющийся субстратом почти для всех реакций метилирования у млекопитающих [2]. Рекомендуемые нормы потребления холина различны для разных категорий населения и колеблются в интервале от 120 мг/сут для младенцев до 520 мг/сут для кормящих матерей [3]. В литературе описаны риски, обусловленные как недостаточным, так и избыточным потреблением холина.
Риски, связанные с недостаточным потреблением холина
Симптоматический дефицит холина встречается редко. Большинство людей получают достаточное количество холина из рациона питания и способны к его биосинтезу [4]. Симптоматический дефицит, как правило, обусловлен определенными заболеваниями или другими косвенными причинами [5]. При дефиците холина снижается доступность фосфатидилхолинов и транспорт жирных кислот из печени, что приводит к накоплению жира в печени [6]. Тяжелый дефицит вызывает повреждение мышц и неалкогольную жировую болезнь печени, которая может перерасти в цирроз [6]. Были предложены и другие одновременно действующие механизмы, объясняющие наблюдаемое повреждение печени. Например, недостаток фосфатидилхолинов приводит к неспособности митохондриальных мембран поддерживать надлежащий электрохимический градиент, который, помимо прочего, необходим для β-окисления жирных кислот. Как результат, снижается скорость метаболизма жиров в печени [7]. Как полагают некоторые исследователи [8], именно митохондриальные дисфункции и оксидативный стресс являются причинами развития неалкогольного жирового гепатоза при дефиците холина и метионина в рационе.
Поскольку дефицит холина снижает выработку SAM, который участвует в метилировании ДНК, это может способствовать канцерогенезу и развитию онкологических заболеваний, также обсуждается связь дефицита холина с сердечно-сосудистыми заболеваниями [9]. Однако обсервационные исследования свободных популяций не показали убедительной связи между низким потреблением холина и сердечно-сосудистыми заболеваниями или большинством видов рака. Исследования связи риска рака простаты с недостаточным потреблением холина дали противоречивые результаты [10, 11]. В то же время общепризнанные рекомендации по повышенному потреблению холина в периоды беременности и грудного вскармливания обусловлены консенсусной позицией относительно рисков его дефицита в эти периоды. Добавка холина является важной стратегией питания беременных женщин для улучшения здоровья плода [12]. Определенные мутации, нарушающие метаболизм холина, или низкое потребление холина матерью обусловливают повышенный риск дефектов нервной трубки плода, тогда как повышенное потребление холина матерью связывают с лучшим нейрокогнитивным развитием у детей [13]. Отмечается, что дефицит холина может быть обусловлен не столько его пониженным содержанием в рационе, сколько низкой биодоступностью. Недостаточное усвоение холина тесно связано с жировыми заболеваниями печени, мышечной дисфункцией [14] и женскими репродуктивными заболеваниями, в частности, концентрация циркулирующего холина снижена у пациенток с синдромом поликистозных яичников [15].
В табл. 1 суммированы некоторые источники, в которых высказываются предположения о ключевом механизме развития патологий, обусловленных дефицитом холина.
Таблица 1.
Механизмы развития некоторых патологий, обусловленных дефицитом холина
| Этиология | Ключевой механизм | Ссылка |
|---|---|---|
| Сердечно-сосудистая патология в сочетании с неалкогольной жировой болезнью печени | Нарушения липидного обмена | [16] |
| Неалкогольная жировая болезнь печени | Дисрегуляция активации макрофагов | [17] |
| Оксидативный стресс | [18] | |
| Митохондриальный оксидативный стресс | [19] | |
| Митохондриальные дисфункции | [20] | |
| Оксидативный стресс, повреждение ДНК | [21] | |
| Нарушение липидного обмена | [22] | |
| Эпигенетика | [23] | |
| Воспаление и фиброз печени | Снижение эффективности фосфорилирования | [24] |
| Мышечная дистрофия | Дефицит метильных групп, нарушение синтеза белков | [25] |
Нарушение липидного обмена, характерное для развития неалкогольной жировой болезни печени, может быть конкретизировано как повышение печеночного липогенеза de novo с преобладанием липопротеинов высокой плотности [26].
Недавно были опубликованы результаты системного исследования влияния дефицита холина на основные регуляторные системы организма [27]. Трансгенные 3xTg-AD и нетрансгенные мыши в течение 9 месяцев получали обогащенную холином (2 г/кг холина битартрата), либо бесхолиновую лабораторную диету. Отметим, что тройная трансгенная мышиная модель AD (3xTg-AD) – единственная модель, в которой наблюдаются оба вида патологий, характерных для человека при болезни Альцгеймера – патология бляшек и патология клубочков. Для этой широко используемой модели характерны три мутации, ассоциированные с болезнью Альцгеймера. Бесхолиновая диета сопровождалась понижением содержания холина в плазме крови, увеличением веса, ухудшением двигательных функций и метаболизма глюкозы, причем у трансгенных мышей эти эффекты были выражены заметнее. Анализ тканей показал наличие патологии сердца и печени, повышенное содержание растворимого и нерастворимого амилоида-β, а также гиперфосфорилированного тау-белка на различных патологических эпитопах в гиппокампе и коре головного мозга трансгенных мышей. Протеомный анализ образцов гиппокампа и плазмы крови показал изменение протеома гиппокампа, связанного с регуляцией функций микротрубочек и постсинаптической мембраны. В плазме крови дефицит холина затрагивал протеом, связанный с уровнем инсулина, функцией митохондрий, воспалительными процессами и метаболизмом фруктозы. Полученные данные свидетельствуют о том, что нормальное потребление холина необходимо как для предотвращения общесистемной органной патологии, так и для снижения тяжести течения болезни Альцгеймера.
Таким образом, достаточное поступление холина важно для поддержания здоровья человека и животных, но в целом роль дефицита холина в рационе питания до настоящего времени не получила исчерпывающей трактовки.
Позитивные метаболические эффекты холина
Холин играет важную роль во многих физиологических процессах, включая физические нагрузки, которые оказывают влияние на синтез нейротрансмиттеров (ацетилхолина), сигнальные функции клеточных мембран (фосфолипиды), транспорт липидов (липопротеины) и метаболизм метильных групп (снижение гомоцистеина), увеличивая потребность в холине как метаболическом субстрате. Физические нагрузки способствуют повышению уровня холина в циркулирующей крови, следовательно, можно полагать, что ресурс доступности свободного холина ассоциирован с толерантностью к физическим нагрузкам. При этом отмечается [28], что напряженная и длительная физическая активность приводит к значимому снижению уровня холина в крови, в таких случаях пероральный прием холина может повысить выносливость. В работе [29] отмечен антиоксидантный эффект холина у пациентов с метаболическим синдромом. Добавка холина к рациону котов, страдающих избыточным весом, оказала благоприятный эффект на состояние печени и повышение доли мышечной массы [30]. Добавка холина в рацион поросят регулирует разнообразие микробиома, активность эпителия кишечника и экспрессию генов цитокинов [31]; авторы исследования предполагают наличие сложных перекрестных связей между уровнем потребления холина, кишечным микробиомом, функцией эпителия и врожденным иммунным ответом, что, в свою очередь, активизирует развитие фолликулов в яичниках. Пожизненное потребление высоких доз холина (5.0 г/кг холинхлорида по сравнению с 1.1 г/кг в группе контрольного питания) значительно снижает риск развития болезни Альцгеймера и связанные с ней когнитивные нарушения [32].
Негативные эффекты избыточного потребления холина
Избыток холина в пище может привести к тромбозу [33], сердечно-сосудистым заболеваниям [34], инсульту [35]. Негативные эффекты избыточного потребления холина связывают с его преобразованием микробиомом кишечника в триметиламин (ТМА), который всасывается в кровь и впоследствии окисляется печеночными ферментами с образованием триметиламин-N-оксида (ТМАО), способствующий патогенезу вышеуказанных заболеваний через гиперактивацию тромбоцитов, усилению образования пенистых клеток и индукцию воспалительных реакций [36]. Пенистыми и менее подвижными макрофаги становятся из-за избытка липидов, они начинают выделять воспалительные сигналы и в итоге гибнут. Повышенная биодоступность холина обусловлена составом кишечного микробиома, в частности, бактериями, продуцирующими ТМА [37]. В то же время рацион с высоким содержанием холина и жиров изменяет состав микроорганизмов кишечника, вырабатывающих ТМА, что, как полагают, опосредовано изменением митохондриальной функции эпителия толстой кишки [38]. Эти данные позволяют предположить, что существует тесное взаимодействие между потреблением холина, микробиомом и функцией кишечника, это приводит к различным режимам активации катаболических путей. В доклинических исследованиях установлено, что повышенное потребление холина приводит к эндотелиальной дисфункции и окислительному повреждению печени [39].
Помимо установления норм потребления холина необходимы соответствующие методы для оценки статуса холина в популяции путем измерения содержания холина и связанных с ним метаболитов в циркулирующей крови.
Содержание свободного холина в плазме крови как показатель физиологического статуса организма
В работе [40] была исследована связь между содержанием холина, бетаина, карнитина и диметилглицина в плазме крови и уровнем кардиометаболических биомаркеров. Показано, что циркулирующие холин, карнитин и диметилглицин связаны с неблагоприятным профилем кардиометаболического риска, в то время как циркулирующий бетаин – напротив, с благоприятным. Несмотря на значительный объем выборки (почти 33 тысячи добровольцев), данные были взяты из 17 различных исследований, так что подходы к аналитическим измерениям не были унифицированы.
В ряде работ отмечается, что состав питания не оказывает определяющего влияния на уровень циркулирующего холина [41]. Это можно объяснить как достаточно низкой абсорбцией холина, так и его активным потреблением тканями. При достаточно длительном голодании (более недели) уровень холина в плазме снижается не более чем на 50% [42]. Стабилизация уровня холина в плазме может осуществляться за счет гидролиза мембранных фосфолипидов и/или за счет биосинтеза [43]. Но даже в случае курсового приема высоких доз холина (3 нед. в дозе 504 мг/кг) его концентрация в сыворотке значимо не повышалась. Авторы исследования [30] объясняют этот факт быстрым и необратимым превращением холина в бетаин и далее делают вывод о том, что содержание холина в циркулирующей крови не отражает дозу его потребления. Поступление холина в организм из внешних источников предлагается оценивать по уровню бетаина в сыворотке или плазме. Влияние голодания и приема пищи на концентрацию холина в плазме исследовано в работе Wiedeman и соавт. [44]. У 40 здоровых добровольцев (мужчин 21, женщин 19, средний возраст 33 года) образцы крови были взяты утром натощак трижды с разницей в 12 дней. У подгруппы из 19 человек (8 мужчин и 11 женщин) был взят один дополнительный образец после завтрака. Средние значения концентрации свободного холина в плазме (±SD) при голодании и после приема пищи составили 6.9 ± 1.7 и 8.3 ± 2.4 мкмоль/л соответственно. В общем случае влияние пищевого потребления холина расценивается как менее значимое в сравнении с метаболическими нарушениями, обусловленными негативными физиологическими процессами.
В работе Gossell-Williams и соавт. [45] средний уровень холина в плазме женщин фертильного возраста оценивается как 10 мкмоль/л, пониженный – 8 мкмоль/л, у беременных женщин установлено более высокое содержание холина в плазме – 14 мкмоль/л. Базовый уровень холина в плазме по данным Holm и соавт. [46] у здоровых добровольцев мужчин (средний возраст 43 года) составил 9.6 мкмоль/л. При этом подтверждается отсутствие не только гендерной, но и возрастной зависимости для содержания холина в плазме. Для пожилых людей среднего возраста 82 года (73–89) размах концентраций холина в плазме мужчин составил 6.7–16.4 мкмоль/л (n = 11) при медианном значении 12.0, у женщин (n = 50) 6.7–14.8 мкмоль/л при медианном значении 11.1 мкмоль/л. При обработке всей выборки без учета гендерной принадлежности (n = 61) медианное значение составило 11.3 мкмоль/л. Если базовые уровни содержания холина в плазме не проявляют гендерной зависимости, то трактовка этого содержания в качестве биомаркера, ассоциированного с определенным физиологическим статусом организма, может быть зависимой от пола. Так, авторы работы [47] установили, что более высокий уровень холина в плазме положительно связан с массой тела и жировой массой, причем эти ассоциации зависят от пола. Считается, что связь уровней триметиламин N-оксида, холина и бетаина с антропометрическими показателями предполагает модулирующее влияние как генетики, так и диеты.
Таким образом, содержание холина в плазме крови, отобранной натощак, может рассматриваться как маркер физиологического статуса организма.
Содержание холина в плазме как показатель риска патологических процессов
Известно, что холин вовлечен в метаболические процессы, связанные с рядом патологических состояний организма [48] и, следовательно, может рассматриваться как биомаркер, уровни которого в диагностических средах могут быть ассоциированы с риском здоровья. Активно исследуются риски, ассоциированные с пониженным содержанием холина в плазме. По данным IOM (Institute of Medicine, США), уровень свободного холина в плазме человека составляет 7–20 мкмоль/л, при этом позиция относительно оптимального уровня остается неоднозначной [49]. Источник [46] сообщает о норме для взрослых людей натощак 7.0–9.3 мкмоль/л. Пониженный уровень холина в плазме может быть ассоциирован с болезнью Альцгеймера и воспалительными процессами [50], а также с резистентностью к инсулину через активацию глюкагона [51] и опасностью развития диабета 2-го типа у пациентов с атеросклерозом [52]. Авторы работы [53] характеризуют холин как один из биомаркеров острого коронарного синдрома, который наряду с тропонинами, натрийуретическими пептидами и маркерами воспаления может служить ранним предиктором сердечно-сосудистых рисков. Уровень холина в сыворотке крови позволяет различить подгруппы высокого и низкого риска инфаркта миокарда у тропонин-положительных пациентов [53]. Холин рассматривается как маркер-кандидат для установления ишемической или неишемической этиологии повышения тропонина. В цитируемом обзоре Danne и Möckel суммированы факторы, связанные с пониженным содержанием холина в плазме, сыворотке и/или клетках крови: хирургические операции, черепно-мозговые травмы, цирроз печени, целиакия, длительное парентеральное питание, длительные изнуряющие физические нагрузки, редкие заболевания (гиперметионемия, синдром Туретта). Патологические состояния, связанные с высокими или возрастающими концентрациями холина в плазме, сыворотке и/или клетках крови: острый коронарный синдром (нестабильная стенокардия и острый инфаркт миокарда), инсульт или церебральная ишемия с каротидными бляшками, болезни почек в терминальной стадии, хронический миелогенный лейкоз, беременность [53].
В доклинических исследованиях показано [54], что при пищевом дефиците холина его содержание в плазме крови животных, получавших витамины группы В, было на 10% выше по сравнению с животными, не получавшими витамины. Можно предположить, что когнитивные дисфункции, обусловленные дефицитом витаминов группы В, связаны в том числе с пониженным уровнем холина в системном кровотоке. Имеется подтверждение этому предположению: у пожилых людей пониженный уровень холина в плазме соответствует более низким когнитивным способностям [55]. Кроме того, установлено положительное влияние холина на прочность костной ткани у людей среднего возраста [56]. Наконец, повышенный уровень холина в сыворотке ассоциирован с благоприятным прогнозом при химиотерапии [57], а содержание холина в плазме является одним из маркеров колоректального рака [58].
Представляют интерес результаты определения свободного холина и транскрипционного фактора Nrf2 в плазме и клетках крови пациентов с хроническими болезнями почек, находящихся на гемодиализе [59]. Транскрипционный фактор Nrf2 обеспечивает антиоксидантный ответ, защищающий клетки при избытке активных форм кислорода (АФК). Уровень холина в плазме крови измеряли методом ВЭЖХ-МС/МС, экспрессию мРНК Nrf2 определяли в изолированных мононуклеарных клетках периферической крови с помощью rt-PCR. Исследования показали, что холин активизирует экспрессию транскрипционного фактора Nrf2.
В работе Schartum-Hansen и соавт. [60] изучена связь содержания холина и его метаболита бетаина в плазме крови с долгосрочным риском развития острого инфаркта миокарда и смертности от всех причин, с учетом статуса курения пациентов со стенокардией. Образцы были получены от 2568 пациентов перед ангиографическим исследованием. Концентрации в плазме холина, но не бетаина, были ниже у курильщиков, при этом холин был положительно связан с С-реактивным белком и тропонином Т у некурящих. Плазменный холин значительно улучшил дискриминацию и реклассификацию при добавлении к установленным факторам сердечно-сосудистого риска, в то время как бетаин плазмы не был связан ни с одной из конечных точек. Повышенное содержание холина в плазме авторы работы [61] рассматривают как индикатор кардиоваскулярного риска, а авторы [62] связывают повышенный риск рака легких с высоким потреблением холина. Во всех случаях холин в плазме как маркер риска достоверно проявляется у некурящих, в то время как для курильщиков достоверная связь отсутствует.
В популяционном исследовании Konstantinov и соавт. [63] в выборке, включающей 7074 мужчин и женщин в возрасте 47–49 и 71–74 лет, изучена связь холина и бетаина в плазме с рядом социально-физиологических и биохимических факторов (курение, физическая активность, индекс массы тела (ИМТ), процент жира в организме, окружность талии, артериальное давление, липиды сыворотки крови, глюкоза). Установлено, что уровень холина и бетаина ниже у женщин, чем у мужчин, и у молодых людей по сравнению с пожилыми. Многомерный анализ показал, что холин положительно коррелирует с триглицеридами, глюкозой, ИМТ, процентом жира, окружностью талии, физической активностью и обратно коррелирует с холестерином ЛПВП и курением. Бетаин был обратно связан с уровнем “плохого” холестерина – ЛПНП, триглицеридов, ИМТ, процентом жира и окружностью талии, систолическим и диастолическим артериальным давлением и курением, но положительно связан с уровнем холестерина ЛПВП и физической активностью. Следовательно, неблагоприятный профиль факторов сердечно-сосудистого риска коррелирует с высокими концентрациями холина и низкими концентрациями бетаина. Тот факт, что холин и бетаин противоположным образом коррелируют с ключевыми компонентами метаболического синдрома, свидетельствует, по мнению авторов, об изменении активности холиндегидрогеназы в митохондриях, что, в свою очередь, может быть связано с изменением митофагии в результате перераспределения этого фермента между наружной и внутренней мембранами митохондрий [64].
Повышенный уровень холина в плазме крови ассоциируется с риском смерти от инфаркта миокарда [65]. Это было установлено при наблюдении порядка 4000 пациентов в возрасте 63 ± 11 лет с риском инфаркта миокарда. Авторы исследования считают возможным рассматривать этот показатель независимо от традиционных факторов риска, но отмечают, что значимость повышенного уровня холина в плазме при сопутствующем повышенном уровне ТМАО существенно повышается. В другом масштабном исследовании с участием почти 7000 человек пациенты ежедневно принимали около 300 мг холина [66]. Уровень холина в плазме составил в среднем 9.61 (8.28–11.1) мкмоль/л, причем у пациентов с хронической фибрилляцией предсердий он был несколько выше – 10.2 (8.82–12.1) мкмоль/л, на основании чего был сделан вывод о вовлеченности метаболизма холина в патогенез фибрилляции предсердий.
Анализируя в совокупности результаты работ Schartum-Hansen и соавт. [60] и Wang и соавт. [65], в которых использован подход, основанный на разделении групп риска на квартили, можно заключить, что уровень холина в плазме, превышающий порог 11–12 мкмоль/л, можно рассматривать как показатель повышенного кардиоваскулярного риска. Ранее было отмечено, что по данным [45] содержание свободного холина в плазме крови женщин в период беременности возрастает и достигает уровня 14 мкмоль/л. Значит ли это, что беременные женщины попадают в зону кардиоваскулярного риска? Это так, но лишь отчасти. Беременность способствует обострению кардиоваскулярного и других рисков, если они имелись ранее. В то же время даже в отсутствие рисков повышенное содержание холина в плазме беременных женщин свидетельствует о мобилизации организма к преодолению нагрузки. Повышенное содержание холина в плазме людей, подвергающихся экстремальным нагрузкам или переживающих период реабилитации после травм и операций [53], также, как мы полагаем, указывает на мобилизацию резервных возможностей организма. Вызовом к мобилизации может стать как повышенная нагрузка в отсутствие патологии, так и развивающееся заболевание.
В исследовании [67] было показано, что содержание холина и его метаболитов в плазме ассоциировано с разными типами сосудистых патологий, что позволяет предположить различную роль холина и его метаболитов в патогенезе сердечно-сосудистых и церебральных заболеваний с вовлечением крупных и мелких сосудов. Некоторые исследователи сердечно-сосудистые заболевания, нарушение функции почек, диабет 2-го типа ассоциируют с уровнем ТМАО в плазме крови [68]. При этом установлено, что не фосфатидилхолин, а именно свободный холин является субстратом для кишечной микробиоты и биопредшественником уремических токсинов – ТМА и ТМАО [69]. Зафиксированы значительные различия в кинетике превращения холина в ТМАО у разных людей. В связи с этим можно предположить, что индивидуальные рекомендации по коррекции кишечного микробиома могут быть сделаны на основании оценки такого показателя, как соотношение концентраций свободного холина и ТМАО в плазме крови. В упрощенном виде схема биотрансформации холина в ТМА и ТМАО представлена на рис. 2. Окисление ТМА происходит с участием печеночного фермента флавин-содержащей монооксигеназы 3 (FMO3), образующейся преимущественно в печени. Нарушение функции этого фермента приводит к триметиламинонурии. Молекулярно-генетическая причина триметиламинонурии заключается в мутации гена, который кодирует FMO3.
Таким образом, холин является достаточно важным диагностическим биомаркером при оценке риска сердечно-сосудистых и некоторых других заболеваний, в связи с этим процедуры определения холина в плазме или сыворотке крови представлены во многих публикациях. Следует отметить, что трудности в исполнении соответствующих методик отмечаются в более поздних работах. Возможно, это связано с ужесточением требований к валидации биоаналитических методик в последнее время. Диагностическая ценность биомаркера определяется наличием достоверной информации о его базовых концентрациях в исследуемых биоматрицах, допустимых вариациях базовых концентраций, соответствующих норме, и, не в последнюю очередь, доступностью валидированных аналитических методик, позволяющих получать совпадающие результаты в разных лабораториях.
Аналитические аспекты
В качестве инструментальных методов при определении холина в пищевых продуктах и биопробах применяются биосенсоры различных типов [70], газовая хроматография [71], капиллярный зонный электрофорез [72], ионная хроматография [73], электрохимические методы [74], ЯМР-спектроскопия [75]. Поскольку холин относится к группе четвертичных аммониевых оснований, которые не способны к образованию летучих производных, использование метода газовой хроматографии даже с предколоночной дериватизацией затруднено. Холин практически не поглощает свет в ультрафиолетовой области спектра, что затрудняет применение ВЭЖХ-УФ. Методом выбора для определения концентраций холина в плазме остается высокоэффективная жидкостная хроматография с тандемным масс-спектрометрическим детектированием (ВЭЖХ-МС/МС). Подавляющее большинство исследований, включающих определение свободного холина в плазме, выполнено методом ВЭЖХ-МС/МС [46, 76 и др.]. При очевидных преимуществах метода ВЭЖХ-МС/МС ограничением к его применению является трудность достижения линейной градуировочной характеристики в биологически обусловленном диапазоне концентраций.
В работе [77] отмечается нелинейность калибровки определения холина методом ВЭЖХ-МС/МС. В цитируемой работе представлены калибровочные кривые, полученные при разработке методики определения четырех метаболически связанных биогенных компонентов плазмы: карнитина, бетаина, ТМАО и холина. Именно для холина калибровочная зависимость была в наибольшей степени нелинейна, поэтому важной задачей при оптимизации методики является достижение линейной градуировочной характеристики. В работе [78] продемонстрировано определение свободного холина в плазме и сыворотке крови методами ВЭЖХ-МС и ЯМР и получены совпадающие результаты. При этом установлено, что в случае приготовления плазмы и сыворотки из одних и тех же образцов крови, содержание холина в сыворотке выше, чем в плазме. Размах концентраций холина в плазме оценен на уровне <5.9–13.1 мкмоль/л, в сыворотке – на уровне <7.1–20.0 мкмоль/л. Интересные данные сравнительного исследования содержания холина в плазме и цельной крови представлены в работе Awwad и соавт. [79]. Цельная кровь перед анализом была гемолизирована. Медиана концентраций холина составила 11.3 мкмоль/л в плазме и 66.6 мкмоль/л в цельной крови соответственно. Наблюдалась положительная корреляция между содержанием холина в цельной крови и плазме (r = 0.42, p ≤ 0.001). Авторы полагают, что концентрации холина в цельной крови отражают его внутриклеточные концентрации, которые, в свою очередь, коррелируют с концентрациями холина в цитозоле. В аналогичных по своим задачам работах [80, 81] авторы не наблюдали значительных различий в содержании холина в цельной крови, стабилизированной ЭДТА или гепарином. При этом в отдельных пробах плазмы, стабилизированных гепарином, стандартное отклонение при определении холина было несколько выше, чем в пробах плазмы, стабилизированных ЭДТА. То обстоятельство, что содержание холина в плазме может быть установлено путем пересчета его концентрации в цельной крови, открывает возможности для малоинвазивного экспресс-тестирования содержания холина в капле крови и, возможно, даже в сухих пятнах крови. В настоящее время подавляющее большинство данных относится к содержанию свободного холина именно в плазме крови, но не в цельной крови или сыворотке.
Возможные причины влияния различных видов пробоподготовки на концентрацию холина в клинических образцах исследованы в работе Ohkawa и соавт. [82]. В качестве одного из факторов, которые могут влиять на изменение концентрации холина во время пробоподготовки, рассматривается аутотаксин (EC 3.1.4.39). Этот фермент идентичен лизофосфолипазе D, которая превращает лизофосфатидилхолин в биоактивный липидный медиатор лизофосфатидную кислоту и холин [83]. Аналитической проблемой является эндогенная природа холина, который поступает в плазму крови из двух источников: пищевое потребление и синтез de novo из фосфатидилхолина [84]. Генетические вариации, обусловливающие разную активность ферментов у разных индивидов, создают значительные трудности в установлении нормы содержания холина в плазме крови на популяционном уровне.
По нашему опыту при определении содержания свободного холина в плазме крови источником ошибок является преаналитический этап (приготовление плазмы крови и условия ее хранения) и последующая подготовка плазмы к инструментальному анализу. Избежать этих ошибок могут помочь некоторые простые рекомендации.
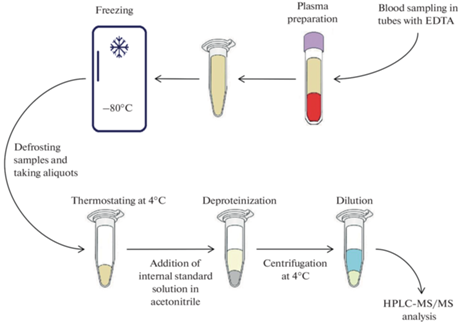
Наиболее эффективным способом подавления активности эстераз, способствующих росту концентрации холина в плазме ex vivo, представляется соблюдение холодового режима на всех этапах хранения и подготовки проб. В наших экспериментах была установлена стабильность свободного холина в плазме крови, стабилизированной ЭДТА, без заморозки в течение суток при температуре не выше 4°С. С учетом литературных данных, оптимальной биоматрицей для определения холина является плазма крови, взятой натощак. Плазму необходимо готовить сразу после отбора крови с использованием охлаждаемой центрифуги и хранить при температуре не выше –18°С. Последовательность операций, предшествующих инструментальному анализу, отражает схема на рис. 3. При использовании масс-спектрометрии в качестве аналитического метода идеальным внутренним стандартом является изотопно-меченый аналог определяемого вещества. Поскольку приобретение или синтез изотопно-меченых стандартов доступны не для всех лабораторий, допускается в качестве внутреннего стандарта использовать заведомо экзогенное вещество, близкое аналиту по структуре и физико-химическим свойствам. Авторы [46] при разработке методики определения холина совместно с бетаином в плазме крови в качестве внутреннего стандарта использовали метформин. Мы в качестве внутреннего стандарта использовали более близкий по структуре к холину мельдоний. Схема подготовки плазмы крови к анализу представлена на рис. 3. Внутренний стандарт (мельдоний) вносили в охлажденный (4°С) ацетонитрил, который добавляли в плазму для депротеинизации. Для разбавления плазмы возможно использование деионизированной воды с добавками 0.1% муравьиной кислоты и формиата аммония в концентрации 5 ммоль/л.
Непосредственное проведение ВЭЖХ-МС/МС анализа не рассматривается нами в качестве источника ошибок при определении концентраций свободного холина в плазме. Единственным требованием является использование охлаждаемого автосамплера для подачи проб. Если не удается обеспечить линейность градуировочной характеристики в области концентраций, превышающих 10 мкмоль/л, проводят разбавление пробы. В этом случае отсутствие влияния разбавления на результат анализа необходимо доказывать в рамках валидационных испытаний. В перспективе переход на определение диагностически значимых биомаркеров, включая холин [85], в сухих пятнах крови позволит отказаться от отбора венозной крови и приготовления плазмы, снимет проблему обеспечения холодового режима на всех этапах подготовки, хранения и транспортировки биопроб. Препятствиями на этом пути остаются нерешенная до настоящего времени проблема правильного учета влияния гематокрита и отсутствие накопленного объема справочных данных о концентрациях биомаркеров в цельной крови.
ЗАКЛЮЧЕНИЕ
Для определения содержания свободного холина оптимальной биоматрицей является плазма крови, стабилизированная ЭДТА. Оптимальным методом для определения холина в плазме является ВЭЖХ-МС/МС в случае успешного преодоления проблемы нелинейности градуировочной характеристики при определении высоких концентраций. Норму содержания холина в плазме для здорового человека можно оценивать в пределах 4–12 мкмоль/л, более высокий уровень холина может расцениваться в качестве дополнительного ресурса при беременности и грудном вскармливании, а также при экстремальных нагрузках. В пожилом возрасте, при болезни Альцгеймера и прочих когнитивных расстройствах, метаболическом синдроме, кардиоваскулярных проблемах и ряде других патологических состояний высокий уровень свободного холина в плазме указывает на риск тяжелого течения и неблагоприятного исхода заболевания.
Прогрессивной стратегией является интерпретация содержания свободного холина в плазме в совокупности с другими показателями, хотя предстоит еще разработать исчерпывающие рекомендации по интерпретации этого биомаркера. Понятно, что интерпретация должна проводиться применительно к конкретным обстоятельствам: предполагаемому или подтвержденному диагнозу, сопутствующим рискам и т.д.
Список литературы
Zeisel SH (2000) Choline: an essential nutrient for humans. Nutrition 16 (7–8): 669–671. https://doi.org/10.1016/s0899-9007(00)00349-x
Goh YO, Cheam G, Wang Y (2021) Understanding Choline Bioavailability and Utilization: First Step Toward Personalizing Choline Nutrition. J Agric Food Chem 69: 10774–10789. https://doi.org/10.1021/acs.jafc.1c03077
Shim E, Park E (2022) Choline intake and its dietary reference values in Korea and other countries: a review. Nutr Res Pract 16: 126–133. https://doi.org/10.4162/nrp.2022.16.S1.S126
Rucker RB, Zempleni J, Suttie JW, McCormick DB (2007) Handbook of vitamins (4th ed). Taylor & Francis 459–477.
Plotnikoff GA, Dobberstein L, Raatz S (2023) Nutritional Assessment of the Symptomatic Patient on a Plant-Based Diet: Seven Key Questions. Nutrients 15: 1387. https://doi.org/10.3390/nu15061387
Osipova D, Kokoreva K, Lazebnik L, Golovanova E, Pavlov C, Dukhanin A, Orlova S, Starostin K (2022) Regression of Liver Steatosis Following Phosphatidylcholine Administration: A Review of Molecular and Metabolic Pathways Involved. Front Pharmacol 13: 797923. https://doi.org/10.3389/fphar.2022.797923
Corbin KD, Zeisel SH (2012) Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression". Current Opinion Gastroenterol 28(2): 159–165. https://doi.org/10.1097/MOG.0b013e32834e7b4b
Chen X, Xue H, Fang W, Chen K, Chen S, Yang W, Shen, T, Chen X, Zhang P, Ling W (2019) Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf 2 mediated antioxidant capacity. Redox Biol 21: 101068.https://doi.org/10.1016/J.REDOX.2018.101068
Dietary reference values for choline (2016) EFSA J 14(8). https://doi.org/10.2903/j.efsa.2016.44842016
Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Zeisel SH, Willett WC, Chan JM (2012) Choline intake and risk of lethal prostate cancer: incidence and survival. Am J Clin Nutrit 96 (4): 855–863. https://doi.org/10.3945/ajcn.112.039784
Han P, Bidulescu A, Barber JR, Zeisel SH, Joshu CE, Prizment AE, Vitolins MZ, Platz EA (2019) Dietary choline and betaine intakes and risk of total and lethal prostate cancer in the Atherosclerosis Risk in Communities (ARIC) Study. Cancer Causes Control 30(4): 343–354. https://doi.org/10.1007/s10552-019-01148-4
Zeisel SH (2006) Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 26: 229–250. https://doi.org/10.1146/annurev.nutr.26.061505.11
Obeid R, Derbyshire E, Schön C (2022) Association between Maternal Choline, Fetal Brain Development, and Child Neurocognition: Systematic Review and Meta-Analysis of Human Studies. Advanc Nutrit 13(6): 2445–2457. https://doi.org/10.1093/advances/nmac082
Buchman AL (2009) The addition of choline to parenteral nutrition. Gastroenterology 137: 119–128. https://doi.org/10.1053/j.gastro.2009.08.010
Troisi J, Cinque C, Giugliano L, Symes S, Richards S, Adair D, Cavallo P, Sarno L, Scala G, Caiazza M, Guida M (2019) Metabolomic change due to combined treatment with myo-inositol, D-chiro-inositol and glucomannan in polycystic ovarian syndrome patients: a pilot study. J Ovar Res 12(1): 25. https://doi.org/10.1186/s13048-019-0500-x
Deprince A, Haas JT, Staels B (2020) Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab 42: 101092. https://doi.org/10.1016/j.molmet.2020.101092
Du X, Wu Z, Xu Y, Liu Y, Liu W, Wang T, Li C, Zhang C, Yi F, Gao L, Liang X, Ma C (2018) Increased Tim-3 expression alleviates liver injury by regulating macrophage activation in MCD-induced NASH mice. Cell Mol Immunol 16(11): 878–886. https://doi.org/10.1038/s41423-018-0032-0
Chen Z, Tian R, She Z, Cai J, Li H (2020) Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biol Med 152: 116−141. https://doi.org/10.1016/j.freeradbiomed.2020.02.025
García-Ruiz C, Fernández-Checa JC (2018) Mitochondrial Oxidative Stress and Antioxidants Balance in Fatty Liver Disease. Hepatol Communicat 2(12): 1425–1439. https://doi.org/10.1002/hep4.1271
Teodoro JS, Rolo AP, Duarte FV, Simões AM, Palmeira CM (2008) Differential alterations in mitochondrial function induced by a choline-deficient diet: Understanding fatty liver disease progression. Mitochondrion 8(5–6): 367–376. https://doi.org/10.1016/j.mito.2008.07.008
Tanaka S, Miyanishi K, Kobune M, Kawano Y, Hoki T, Kubo T, Hayashi T, Sato T, Sato Y, Takimoto R, Kato J (2013) Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J Gastroenterol 48(11): 1249–1258. https://doi.org/10.1007/s00535-012-0739-0
Pei K, Gui T, Kan D, Feng H, Jin Y, Yang Y, Zhang Q, Du Z, Gai Z, Wu J, Li Y (2020) An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. BioMed Res Int 1–12: 4020249. https://doi.org/10.1155/2020/4020249
Ashraf NU, Altaf M (2018) Epigenetics: An emerging field in the pathogenesis of nonalcoholic fatty liver disease. Mutat Res Rev Mut Res 778: 1–12. https://doi.org/10.1016/j.mrrev.2018.07.002
Yoo N, Jeon S, Nam Y, Park Y-J, Won SB, Kwon Y (2015) Dietary Supplementation of Genistein Alleviates Liver Inflammation and Fibrosis Mediated by a Methionine-Choline-Deficient Diet in db/db Mice. J Agricult Food Chem 63(17): 4305–4311. https://doi.org/10.1021/acs.jafc.5b00398
Alves FM, Caldow MK, Trieu J, Naim T, Montgomery MK, Watt MJ, Lynch GS, Koopman R (2019) Choline administration attenuates aspects of the dystrophic pathology in mdx mice. Clin Nutrit Exp 24: 83–91. https://doi.org/10.1016/j.yclnex.2018.12.005
Payne F, Lim K, Girousse A, Brown RJ, Kory N, Robbins A, Xue Y, Sleigh A, Cochran E, Adams C, Dev Borman A, Russel-Jones D, Gorden P, Semple RK, Saudek V, O’Rahilly S, Walther TC, Barroso I, Savage DB (2014) Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A 111(24): 8901–8906. https://doi.org/10.1073/pnas.1408523111
Dave N, Judd J M, Decker A,Winslow W, Sarette P, Villarreal Espinosa O, Tallino S, Bartholomew SK, Bilal A, Sandler J, McDonough I,Winstone J K, Blackwood E A, Glembotski C, Karr T, Velazquez R (2023) Dietary choline intake is necessary to prevent systems-wide organ pathology and reduce Alzheimer’s disease hallmarks. Aging Cell 22: e13775. https://doi.org/10.1111/acel.13775
Penry JT, Manore MM (2008) Choline: An Important Micronutrient for Maximal Endurance-Exercise Performance? Int J Sport Nutr Exerc Metab 18(2): 191–203. https://doi.org/10.1123/ijsnem.18.2.191
Dibella M, Thomas MS, Alyousef H, Millar C, Blesso C, Malysheva O (2020) Choline intake as supplement or as a component of eggs increases plasma choline and reduces interleukin-6 without modifying plasma cholesterol in participants with metabolic syndrome. Nutrients12: 1e. https://doi.org/10.3390/nu12103120
Rankovic A, Godfrey H, Grant CE, Shoveller AK, Bakovic M, Kirby G (2023) Serum metabolomic analysis of the dose-response effect of dietary choline in overweight male cats fed at maintenance energy requirements. PLoS One 18(1): e0280734. https://doi.org/10.1371/journal.pone.0280734
Zhan X, Fletcher L, Huyben D, Cai H, Dingle S, Qi N, Huber L-A, Wang B, Li J (2023) Choline supplementation regulates gut microbiome diversity, gut epithelial activity, and the cytokine gene expression in gilts. Front Nutr 10: 1101519. https://doi.org/10.3389/fnut.2023.1101519
Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow W, Oddo S (2019) Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 18: e13037. https://doi.org/10.1111/acel.13037
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165: 111–124. https://doi.org/10.1016/j.cell.2016.02.011
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63. https://doi.org/10.1038/nature09922
Zhu W, Romano KA, Li L, Buffa JA, Sangwan N, Prakash P (2021) Gut microbes impact stroke severity via the trimethylamine N-Oxide pathway. Cell Host Microbe 29: 1199–1208. https://doi.org/10.1016/j.chom.2021.05.002
Yang S, Li X, Yang F, Zhao R, Pan X, Liang J (2019) Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol 10: 1360. https://doi.org/10.3389/fphar.2019.01360
Romano KA, Vivas EI, Amador-Noguez D, Rey FE (2015) Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite Trimethylamine-N-Oxide. mBio 6: e02481-14. https://doi.org/10.1128/mBio.02481-14
Yoo W, Zieba JK, Foegeding NJ, Torres TP, Shelton CD, Shealy NG (2021) High-Fat Diet–Induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 373: 813–818. https://doi.org/10.1126/science.aba3683
Ren D, Liu Y, Zhao,Y, Yang X (2016) Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem Toxicol 94: 203–212. https://doi.org//10.1016/j.fct.2016.06.004
Pan XF, Yang JJ, Shu XO, Moore SC, Palmer ND, Guasch-Ferré M, Herrington DM, Harada S, Eliassen H, Wang TJ, Gerszten RE, Albanes D, Tzoulaki I, Karaman I, Elliott P, Zhu H, Wagenknecht LE, Zheng W, Cai H, Cai Q, Matthews CE, Menni C, Meyer KA, Lipworth LP, Ose J, Fornage M, Ulrich CM, Yu D (2021) Associations of circulating choline and its related metabolites with cardiometabolic biomarkers: an international pooled analysis. Am J Clin Nutr 114(3): 893–906. https://doi.org/10.1093/ajcn/nqab152
Mujica M, Lewis E, Jacobs R, Letourneau N, Bell R, Field C, Lamers Y (2020) Plasma Free Choline Concentration Did Not Reflect Dietary Choline Intake in Early and Late Pregnancy: Findings from the APrON Study. Curr Dev Nutr 4(Suppl 2): 1825.https://doi.org/10.1093/cdn/nzaa067_052
Zeisel SH (2010) Choline. In: Coates PM, Betz JM, Blackman MR (eds). Encyclopedia of Dietary Supplements. 2nd ed. London and New York. Informa Healthcare 136–143.
Finglas PM (2000) Dietary Reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Trends Food Sci Technol 11(8): 296–297. https://doi.org/10.1016/s0924-2244(01)00010-3
Wiedeman AM, Dyer RA, Green TJ, Xu Z, Barr SI, Innis SM, Kitts DD (2018) Variations in plasma choline and metabolite concentrations in healthy adults. Clin Biochem 60: 77–83. https://doi.org/10.1016/j.clinbiochem.2018.08.002
Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S (2005) Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J 54(6): 355–359. https://doi.org/10.1590/s0043-31442005000600002
Holm PI, Ueland PM, Kvalheim G, Lien EA (2003) Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 49: 286–294. https://doi.org/10.1373/49.2.286
Mlodzik-Czyzewska MA, Malinowska AM, Szwengiel A, Chmurzynska A (2002) Associations of plasma betaine, plasma choline, choline intake, and MTHFR polymorphism (rs1801133) with anthropometric parameters of healthy adults are sex-dependent. J Hum Nutr Diet 35: 701– 712. https://doi.org/10.1111/jhn.13046
Arias N, Arboleya S, Allison J, Kaliszewska A, Higarza SG, Gueimonde M, Arias JL (2020) The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 12 (8): 2340. https://doi.org/10.3390/nu12082340
Choline – dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline – NCBI bookshelf n.d.
Siddiqui A, Shah Z, Jahan RN, Othman I, Kumari Y (2021) Mechanistic role of boswellic acids in Alzheimer’s disease: emphasis on anti-inflammatory properties. Biomed Pharmacother 144: 112250. https://doi.org/10.1016/J.BIOPHA.2021.112250
Wu G, Zhang L, Li T, Zuniga A, Lopaschuk GD, Li L, Jacobs RL, Vance DE (2013) Choline supplementation promotes hepatic insulin resistance in phosphatidylethanolamine N-methyltransferase-deficient mice via increased glucagon action. J Biol Chem 288(2): 837–847. https://doi.org/10.1074/jbc.M112.415117
Dibaba DT, Johnson KC, Kucharska-Newton AM, Meyer K, Zeisel SH, Bidulescu A (2020) The Association of Dietary Choline and Betaine with the Risk of Type 2 Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 43(11): 2840–2846. https://doi.org/10.2337/dc20-0733
Danne O, Möckel M (2010) Choline in acute coronary syndrome: an emerging biomarker with implications for the integrated assessment of plaque vulnerability. Expert Rev Mol Diagnost 10(2): 159–171. https://doi.org/10.1586/erm.10.2
Van Wijk N, Watkins C, Böhlke M, Maher T, Hageman R, Kamphuis P, Wurtman R (2012) Plasma choline concentration varies with different dietary levels of vitamins B6, B12 and folic acid in rats maintained on choline-adequate diets. Br J Nutrit 107(10): 1408–1412. https://doi.org/10.1017/S0007114511004570
Nurk E, Refsum H, Bjelland I, Drevon CA, Tell GS, Ueland PM, Vollset SE, Engedal K, Nygaard HA, Smith DA (2013) Plasma free choline, betaine and cognitive performance: the Hordaland Health Study. Br J Nutrit 109(3): 511–519. https://doi.org/10.1017/S0007114512001249
Øyen J, Gjesdal CG, Karlsson T, Svingen GF, Tell GS, Strand E, Drevon CA, Vinknes KJ, Meyer K, Ueland PM, Nygård O (2017) Dietary Choline Intake Is Directly Associated with Bone Mineral Density in the Hordaland Health Study. J Nutrition 147(4): 572–578. https://doi.org/10.3945/jn.116.243006
Watson GA, Sanz-Garcia E, Zhang W-J, Liu ZA, Yang SC, Wang B, Liu S, Kubli S, Berman H, Pfister T, Genta S, Spreafico A, Hansen AR, Bedard PL, Lheureux S, Razak A, Cescon D, Butler MO, Xu W, Chen E (2022) Increase in serum choline levels predicts for improved progression-free survival (PFS) in patients with advanced cancers receiving pembrolizumab. J Immun Therapy Cancer 10(6): e004378 https://doi.org/10.1136/jitc-2021-004378
Li S, Guo B, Song J, Deng X, Cong Y, Li P, Zhao K, Liu L, Xiao G, Xu F, Ye Y, Zhao Z, Yu M, Xu Y, Sang J, Zhang J (2012) Plasma choline-containing phospholipids: potential biomarkers for colorectal cancer progression. Metabolomics 9(1): 202–212. https://doi.org/10.1007/s11306-012-0439-z
Mafra D, Cardozo L, Ribeiro-Alves M, Bergmane P, Shiels PG, Stenvinkel P (2022) Short Report: Choline plasma levels are related to Nrf2 transcriptional expression in chronic kidney disease? Clin Nutrition ESPEN 50: 318–321. https://doi.org/10.1016/j.clnesp.2022.06.008
Schartum-Hansen H, Pedersen ER, Svingen GF, Ueland P M, Seifert R, Ebbing M, Strand E, Bleie Ø, Nygård O (2014) Plasma choline, smoking, and long-term prognosis in patients with stable angina pectoris. Eur J Prevent Cardiol 22(5): 606–614. https://doi.org/10.1177/2047487314524867
Garbuzenko DV, Belov DV (2021) Non-alcoholic fatty liver disease as an independent factor of cardiometabolic risk of cardiovascular diseases. Exp Clin Gastroenterol 10: 22–34. https://doi.org/10.31146/1682-8658-ecg-194-10-22-34
Ying J, Rahbar MH, Hallman DM, Hernandez LM, Spitz MR, Forman MR, Gorlova OY (2013) Associations between Dietary Intake of Choline and Betaine and Lung Cancer Risk. PLoS One 8(2): e54561. https://doi.org/10.1371/journal.pone.0054561
Konstantinova SV, Tell GS, Vollset SE, Nygård O, Bleie Ø, Ueland PM (2008) Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr 138(5): 914–920. https://doi.org/10.1093/jn/138.5.914
Park S, Choi SG, Yoo SM, Son JH, Jung YK (2014) Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy. Autophagy 10(11): 1906–1920. https://doi.org/10.4161/auto.32177
Wang, Z, Tang WHW, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL (2014) Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 35(14): 904–910. https://doi.org/10.1093/eurheartj/ehu002
Zuo H, Svingen GFT, Tell GS, Ueland PM, Vollset SE, Pedersen ER, Ulvik A, Meyer K, Nordrehaug JE, Nilsen DWT, Bønaa KH, Nygård O (2018) Plasma Concentrations and Dietary Intakes of Choline and Betaine in Association with Atrial Fibrillation Risk: Results From 3 Prospective Cohorts with Different Health Profiles. J Am Heart Assoc 7(8): e008190. https://doi.org/10.1161/jaha.117.008190
Roe AJ, Zhang S, Bhadelia RA, Johnson EJ, Lichtenstein AH, Rogers GT, Rosenberg IH, Smith CE, Zeisel SH, Scott TM (2017) Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am J Clin Nutr 105(6): 1283–1290. https://doi.org/10.3945/ajcn.116.137158
Gessner A, di Giuseppe R, Koch M, Fromm MF, Lieb W, Maas R (2020) Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: distribution and correlates in the population-based PopGen cohort. Clin Chem Labor Med 58(5): 733–740. https://doi.org/10.1515/cclm-2019-1146
Cho CE, Aardema NDJ, Bunnell ML, Larson DP, Aguilar SS, Bergeson JR, Malysheva OV, Caudill MA, Lefevre M (2020) Effect of Choline Forms and Gut Microbiota Composition on Trimethylamine-N-Oxide Response in Healthy Men. Nutrients 12(8): 2220. https://doi.org/10.3390/nu12082220
Rahimi P, Joseph Y (2019) Enzyme-based biosensors for choline analysis: A review. Trends Analyt Chem 110: 367–374. https://doi.org/10.1016/j.trac.2018.11.035
Saucerman JR, Winstead CE, Jones TM (1984) Quantitative Gas Chromatographic Headspace Determination of Choline in Adult and Infant Formula Products. J Assoc Anal Chem 67(5): 982–985. https://doi.org/10.1093/jaoac/67.5.982
Zhang L, LiuY, Chen G (2004) Simultaneous determination of allantoin, choline and l-arginine in Rhizoma Dioscoreae by capillary electrophoresis. J Chromat A 1043(2): 317–321. https://doi.org/10.1016/j.chroma.2004.06.003
Lin L, Li R, Wang L, Qiu Y (2018) Determination of choline, putrescine and cadaverine in boletus by ion chromatography with suppressed conductivity detection. Chin J Chromat 36(11): 1189. https://doi.org/10.3724/sp.j.1123.2018.06027
Shadlaghani A, Farzaneh M, Kinser D, Reid RC (2019) Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes. Sensors 19(3): 447. https://doi.org/10.3390/s19030447
Garcia E, Shalaurova I, Matyus SP, Wolak-Dinsmore J, Oskardmay DN, Connelly MA (2022) Quantification of choline in serum and plasma using a clinical nuclear magnetic resonance analyzer. Clin Chim Acta 524: 106–112. https://doi.org/10.1016/j.cca.2021.11.031
Ocque AJ, Stubbs JR, Nolin TD (2015) Development and validation of a simple UHPLC-MS/MS method for the simultaneous determination of trimethylamine N-oxide, choline, and betaine in human plasma and urine. J Pharm Biomed Anal 109: 128–135. https://doi.org/10.1016/j.jpba.2015.02.040
Rox K, Rath S, Pieper DH, Vital M, Brönstrup M (2021) A simplified LC-MS/MS method for the quantification of the cardiovascular disease (CVD) biomarker trimethylamine-Noxide (TMAO) and its precursors. J Pharm Anal 11(4): 523–528. https://doi.org/10.1016/j.jpha.2021.03.007
Garcia E, Shalaurova I, Matyus SP, Wolak-Dinsmore J, Oskardmay DN, Connelly MA (2022) Quantification of choline in serum and plasma using a clinical nuclear magnetic resonance analyzer. Clin Chim Acta 524: 106–112. https://doi.org/10.1016/j.cca.2021.11.031
Awwad HM, Kirsch SH, Geise J, Obeid R (2014) Measurement of concentrations of whole blood levels of choline, betaine, and dimethylglycine and their relations to plasma levels. J Chromat B 957: 41–45. https://doi.org/10.1016/j.jchromb.2014.02.030
Yue B, Pattison E, Roberts WL, Rockwood AL, Danne O, Lueders C, Möckel M (2008) Choline in Whole Blood and Plasma: Sample Preparation and Stability. Clin Chem 54(3): 590–593. https://doi.org/10.1373/clinchem.2007.094201
Kaplan A (2021) Preparation, Storage, and Characteristics of Whole Blood, Blood Components, and Plasma Derivatives. Transfus Med 11: 59–89. https://doi.org/10.1002/9781119599586.ch5
Ohkawa R, Kurano M, Sakai N, Kishimoto T, Nojiri T, Igarashi K, Hosogaya S, Ozaki Y, Dohi T, Miyauchi K, Daida H, Aoki J, Okubo S, Ikeda H, Tozuka M, Yatomi Y (2018) Measurement of plasma choline in acute coronary syndrome: importance of suitable sampling conditions for this assay. Scient Rep 8(1): 4725. https://doi.org/10.1038/s41598-018-23009-x
Burdeynaya AL, Afanasyeva OI, Klesareva EA, Tmoyan NA, Razova OA, Afanasyeva MI, Ezhov MV, Pokrovsky SN (2021) Role of inflammation, autotaxin and lipoprotein (a) in degenerative aortic valve stenosis in patients with coronary artery disease. Cardiovasc Ther Prevent 20(2): 2598. https://doi.org/10.15829/1728-8800-2021-2598
Klatt KC (2023) Choline and phosphatidylcholine. Encycloped Human Nutrit 162–174. https://doi.org/10.1016/b978-0-12-821848-8.00020-2
Liu L, Jin X, Wu Y, Yang M, Xu T, Li X, Ren J, Yan LL (2020) A Novel Dried Blood Spot Detection Strategy for Characterizing Cardiovascular Diseases. Front Cardiovasc Med 7: 542519. https://doi.org/10.3389/fcvm.2020.542519
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова