Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 5, стр. 572-587
Экспрессия молекул Tim-3 и CD9 на натуральных киллерах (NK) и Т-лимфоцитах с функциями NK (NKT) периферической крови в разные сроки физиологической беременности
Е. Г. Орлова 1, *, О. А. Логинова 1, О. Л. Горбунова 1, Н. В. Каримова 2, С. В. Ширшев 1
1 Институт экологии и генетики микроорганизмов УрО РАН
Пермь, Россия
2 ООО Централизованная клинико-диагностическая лаборатория
Пермь, Россия
* E-mail: orlova_katy@mail.ru
Поступила в редакцию 06.03.2023
После доработки 21.03.2023
Принята к публикации 22.03.2023
- EDN: XRCARE
- DOI: 10.31857/S0869813923050072
Аннотация
При физиологически протекающей беременности натуральные киллеры (NK) и Т-лимфоциты с функциями NK (NKT) являются ведущими эффекторами иммунной толерантности организма матери к полуаллогенному плоду, а также выполняют фетотрофическую функцию. Молекулы Tim-3 (T-cell Ig and mucin domain-containing protein 3) и CD9 играют критическую роль в реализации иммунорегуляторной и фетотрофической функций NK и NKT, однако их экспрессия на клетках периферической крови не изучена. Цель работы – исследовать экспрессию Tim-3, CD9 на субпопуляциях NK и NKT периферической крови в разные сроки физиологически протекающей беременности. Объектом исследования являлась периферическая кровь условно-здоровых женщин в I и III триместрах физиологически протекающей беременности. Группу сравнения составили условно-здоровые небеременные женщины в I фазе менструального цикла. Экспрессию молекул Tim-3, CD9 анализировали методом проточной цитофлюориметрии на регуляторных NK (CD16–CD56bright) и NKT (CD16–CD56+), цитотоксических NK (CD16+CD56dim/–) и NKT (CD16+CD56+). Установлено, что в I триместре беременности количество и соотношение регуляторных и цитотоксических NK и NKT не менялось. Экспрессия Tim-3 увеличивалась на всех субпопуляциях NK и NKT за исключением цитотоксических CD16+CD56dimNK. Экспрессия CD9 возрастала на всех субпопуляциях NK, а на NKT не отличалась от небеременных. При этом на регуляторных NK и NKT в I триместре беременности выявлена прямая корреляция экспрессии CD9 и Tim-3. В III триместре количество регуляторных CD16–CD56brightNK увеличивалось, а цитотоксических CD16+CD56dimNK и регуляторных CD16–CD56+NKТ снижалось по сравнению с небеременными. Количество CD16+CD56–NK не менялось по триместрам беременности. Экспрессия Tim-3 усиливалась на всех субпопуляциях NK и цитотоксических NKТ, а CD9 повышалась только на регуляторных NK. Таким образом, экспрессия молекул Tim-3 и CD9 на разных субпопуляциях NK и NKT менялась по триместрам, что играет важную роль в регуляции их фенотипа и функций при беременности.
ВВЕДЕНИЕ
Натуральные киллеры (NK) и T-лимфоциты с функциями NK (NKТ) при беременности играют критическую роль в формировании иммунной толерантности организма матери к полуаллогенному плоду, а также выполняют фетотрофическую функцию [1, 2]. NK-клетки относятся к эффекторам врожденного иммунитета [1, 2]. Более 90% NK периферической крови имеют фенотип CD16+CD56dim и обладают высокой цитотоксической активностью в отношении опухолевых и вирус-инфицированных клеток [1, 3]. И лишь около 10% от общего числа NK-клеток имеют регуляторный фенотип CD16–CD56bright, основной функцией которых является продукция широкого спектра цитокинов [1, 3]. При беременности изменение гормонального фона индуцирует трансформацию фенотипа NK периферической крови с цитотоксического на регуляторный и миграцию CD16–CD56brightNK в матку, где они становятся доминирующей лимфоидной популяцией, о чем свидетельствует сходный паттерн хемокиновых рецепторов периферических и децидуальных CD16–CD56brightNK [1, 3]. Количество CD16+CD56dim NK периферической крови, их цитотоксический потенциал, продукция интерферона-гамма (IFN-gamma) при беременности снижаются, но увеличивается число интерлейкин (IL)-10-продуцирующих NK, что особенно выражено в I триместре беременности [4]. Повышенное содержание цитотоксических CD16+CD56dimNK в периферической крови у женщин ассоциировано с нарушением фертильности и спонтанными абортами [1, 3]. Исследования последних лет показали, что в периферической крови присутствует также субпопуляция цитотоксических истощенных CD16+CD56–NK, которая характеризуется сниженной цитолитической и секреторной активностью [5–7]. Содержание CD16+CD56–NK увеличивается при тяжелых вирусных инфекциях и связано со снижением противовирусной защиты, однако изменения их количества и функций при физиологической беременности не изучены [5–7].
В ранние сроки беременности количество децидуальных NK увеличивается до 70–90% от общего числа лимфоцитов в матке [8, 9]. Децидуальные NK продуцируют ангиогенные факторы (VEGF, PLGF), моделируют рост спиральных артерий, инвазию трофобласта, формирование иммунной толерантности в зоне фето-плацентарного контакта [1, 2, 10]. Секретируя иммуносупрессорные цитокины – IL-10, трансформирующий фактор роста (TGF-1beta), децидуальные NK индуцируют формирование адаптивных Т-регуляторных клеток (aTreg) из наивных CD4+ T-лимфоцитов, которые за счет контактных взаимодействий и продукции IL-10, TGF-1 beta подавляют цитотоксические реакции против трофобласта [1, 2, 10, 11].
Децидуальные CD56brightCD16–NK отличаются от периферических регуляторных CD56brightCD16NK высокой экспрессией молекул Tim-3 и CD9, которые регулируют цитотоксическую и миграционную активность, продукцию ангиогенных факторов [8, 9, 12–19]. “Сheck-point” молекула Tim-3 (T-cell Ig and mucin domain-containing protein 3) присутствует на большинстве лимфоидных клеток, однако NK обладают наибольшей экспрессией Tim-3, которая повышается при их трансформации в децидуальные [13, 14]. Экспрессия Tim-3 усиливается в ответ на активацию клеток и ограничивает продукцию провоспалительных цитокинов, дегрануляцию, цитотоксичность, повышает чувствительность к индуцированному апоптозу [15, 16]. Лигандом для Tim-3 является галектин-9 (Gal-9), уровень которого в периферической крови нарастает при беременности [14], поскольку Gal-9 активно продуцируется клетками трофобласта, Treg [17]. Молекула CD9 относится к семейству тетраспонинов и регулирует адгезию и трансэндотелиальную миграцию лейкоцитов, а также экспрессию и активность других адгезионных молекул [19, 20]. Экспрессия CD9 изучена на децидуальных NK, но лишь в единичных исследованиях выявлена на NK и других популяциях лимфоцитов периферической крови при беременности [20, 21]. Лигандами для CD9, в том числе, являются продуцируемые клетками трофобласта ассоциированные с беременностью гликопротеины (“pregnancy specific glycoproteins”, PSG), концентрация которых нарастает в периферической крови пропорционально сроку беременности [22]. Взаимодействие CD9 с PSG регулирует продукцию цитокинов лейкоцитами в матке [22]. Все вышесказанное подтверждает значимость молекул Tim-3 и CD9 для контроля функций NK, однако их экспрессия разными популяциями NK периферической крови при беременности не исследована.
NKT представляют гетерогенную минорную популяцию Т-лимфоцитов периферической крови, выделение которой основано на экспрессии Т-клеточного рецептора (TCR) и молекул NK клеток – CD56, CD16 [23–25]. К NKT относятся как инвариантные (i) NKT, экспрессирующие инвариантный TCR и распознающие гликолипидные антигены, ассоциированные с молекулой CD1d, которая высоко экспрессируется клетками трофобласта, так и NKТ-подобные клетки, включающие CD1d-независимые αβTCR Т-лимфоциты [26]. Критическая роль NKT в регуляции иммунореактивности определяется способностью к быстрой и массивной продукции цитокинов (INF-gamma, TNF-alpha, IL-4, IL-10), направляющих развития иммунного ответа по клеточно-опосредованному или гуморальному типу [25–27]. Известно, что в ранние сроки физиологической беременности меняется субпопуляционный состав и количество NKT в периферической крови [25, 28]. Выявлено повышение экспрессии активационного маркера CD69, продукции IL-4 и, напротив, снижение цитотоксичности и секреции INF-gamma [28–30]. Избыточное количество NKT и продукция INF-gamma в периферической крови в ранние сроки беременности приводит к спонтанным абортам [25, 31, 32]. NKT не присутствуют в эндометрии вне беременности [33], но в I триместре количество CD56+CD16– NKT в матке значительно увеличивается [29], а к родам снижается [32]. В зоне фето-плацентарного контакта NKT выполняют фетотрофическую и регуляторную функцию, продуцируют INF-gamma и гранулоцитарно-макрофагальный колониестимулирующий фактор (GM-CSF), стимулируют рост спиральных артерий и развитие децидуальных макрофагов, пролиферацию децидуальных NK in situ, подавляют клеточноопосредованные реакции [29]. Возможность миграции CD56+CD16–NKT периферической крови в матку при беременности остается не изученной. Развитие NKT происходит в тимусе, продуктивная функция которого снижается при беременности вследствие стероид-индуцированной инволюции. В единичных работах показана экспрессия Tim-3 [17, 34], CD9 [35] на NKT периферической крови, однако их роль в изменении фенотипа и функций NKT при беременности не исследована.
При физиологической беременности иммунореактивность организма матери меняется по триместрам [36]. Первый триместр характеризуется наибольшей частотой спонтанных абортов, что связано с началом экспрессии молекул главного комплекса гистосовместимости клетками трофобласта и “лютео-плацентарной сменой” [37]. В третьем триместре изменения иммунореактивности организма матери связаны с подготовкой к родам. Все высказанное подтверждает значимость и актуальность изучения трансформации фенотипа эффекторов иммунной толерантности по триместрам беременности. Цель работы – изучить экспрессию Tim-3 и CD9 на регуляторных и цитотоксических субпопуляциях NK, NKT периферической крови женщин в I и III триместрах физиологически протекающей беременности.
МЕТОДЫ ИССЛЕДОВАНИЯ
Объекты исследования
Исследовали периферическую кровь условно-здоровых небеременных (I фаза менструального цикла) и женщин с физиологически протекающей беременностью в I и III триместрах. Критериями включения являлось: наличие одной и более успешно завершившихся беременностей; отсутствие патологий беременности в прошлом и настоящем; отсутствие острых и хронических соматических, эндокринных, аутоиммунных, генетических заболеваний; отрицание диет, приема контрацептивных и гормональных, противовоспалительных или антибактериальных препаратов; наличие добровольного информированного согласия на использование биологического материала. Клиническая и демографическая характеристика участников исследования представлена в табл. 1. Исследуемые группы не отличались по возрастному составу.
Анализ фенотипа клеток
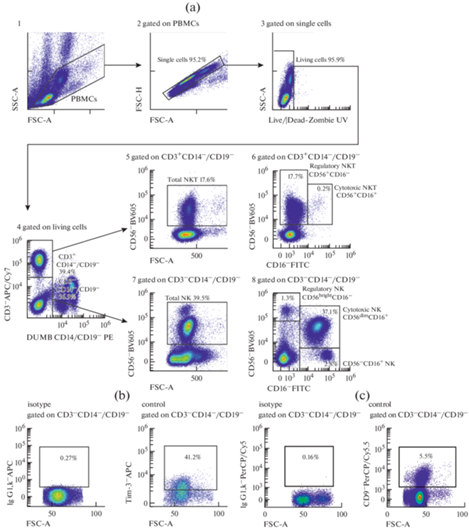
Венозную кровь забирали из локтевой вены (утром, натощак) объемом 2 мл в вакуумные пробирки с этилендиаминтетрауксусной кислотой (ЭДТА). Мононуклеарные клетки периферической крови (PBMC) выделяли методом седиментации в градиенте плотности фиколл-урографина (1.077 г/см3) по стандартной методике [38]. Затем PBMC дважды отмывали в фосфатно-солевом буфере (ФСБ), содержащим 2 мМ ЭДТА и 0.1% бычьего сывороточного альбумина (БСА). Фенотип клеток (1 × 106 клеток в пробе) анализировали методом проточной цитофлюориметрии на проточном цитометре “CytoFlex S” (BeckmanCoulter, США) с использованием программы “CytExpert 2.0” (BeckmanCoulter, США). Окрашивание моноклональными антителами проводили согласно инструкции производителя (табл. 2). Анализировали не менее 100 000 событий в каждой пробе. Для уменьшения неспецифического связывания и адгезии клеток окрашивание проводили в ФСБ, содержащем 2 мМ ЭДТА и 0.1% БСА, без использования консервантов. Для контроля неспецифического связывания и выделения негативного по флюоресценции окна использовали соответствующие изотипические и негативные контроли. Стратегия гейтирования представлена на рис. 1. В гейте PBMC оценивали: общее количество (total) NK-клеток как процент CD56+-клеток в гейте CD3/CD14/CD19-негативных мононуклеаров [39]. Субпопуляции NK определяли по коэкспрессии молекул CD56 и CD16 в гейте CD3/CD14/CD19 негативных мононуклеаров: регуляторные (regulatory) NK – CD16–CD56bright, цитотоксические (cytotoxic) NK – CD16+CD56dim и CD16+CD56– [17]. Общее количество (total) NKT оценивали как процент CD56+-клеток в гейте CD3+-лимфоцитов; регуляторные NKT – как процент CD16–CD56+, а цитотоксические NKT – как процент CD16+CD56+-клеток в гейте CD3+лимфоцитов (рис. 1) [40]. Экспрессию молекул Tim-3, CD9 изучали на разных субпопуляциях NK и NKT (рис. 1).
Таблица 2.
Характеристика моноклональных антител
| Моноклональные антитела | Флюорохром | Клон | Изотип | Компания-производитель |
|---|---|---|---|---|
| Live/Dead | Zombie UV™ | BioLegend | ||
| CD3 | APC/Cy7 | UCHT1 | Mouse/IgG1, κ | BioLegend |
| CD14 | PE | M5E2 | Mouse/IgG2a, κ | BioLegend |
| CD19 | PE | HIB19 | Mouse/IgG1, κ | BioLegend |
| CD56 (NCAM) | Brilliant Violet 605™ | HCD56 | Mouse/IgG1, κ | BioLegend |
| CD16 | FITC | 3G8 | Mouse/IgG1, κ | BioLegend |
| Tim-3 (CD366) | APC | F38-2E2 | Mouse/IgG1, κ | BioLegend |
| CD9 | PerCP/Cyanine5.5 | HI9a | Mouse/IgG1, κ | BioLegend |
| Изотипический контроль | APC | MOPC-21 | Mouse/IgG1, κ | BioLegend |
| Изотипический контроль | PerCP/Cyanine5.5 | MOPC-21 | Mouse/IgG1, κ | BioLegend |
Рис. 1.
Стратегия гейтирования NK и NKT периферической крови. (a) 1 – выделение гейта PBMC по параметрам площади прямого (FSC-A) и высоты бокового (SSC-H) светорассеивания; 2 – дискриминация слипшихся клеток (дуплетов) по параметрам площади и высоты прямого светорассеивания (FSC-A/FSC-H); 3 – определение живых клеток LIVE/DEAD-ZOMBIE UV окрашиванием, 4 – выделение популяций CD3– и CD3+-клеток в PBMC гейте, 5 – определение общего количества (total) NKT как процента CD56+-клеток в гейте CD3+-лимфоцитов; 6 – выделение регуляторной субпопуляции (regulatory) NKT как процента CD16–CD56+ и цитотоксической субпопуляции (cytotoxic) NKT как процента CD16+CD56+ клеток в гейте CD3+лимфоцитов; 7 – определение общего количества (total) NK как процента CD56+-клеток в гейте CD3/CD14/CD19-негативных PBMC; 8 – определение регуляторной субпопуляции (regulatory) NK как процента CD16–CD56bright и цитотоксических субпопуляций (cytotoxic) NK как процента CD16+CD56dim и CD16+CD56– в гейте CD3/CD14/CD19-негативных PBMC; (b, c) – оценка неспецифического связывания с использованием соответствующих изотипических МКА (isotype) для определения уровня экспрессии молекул Tim-3, CD9. На рис. 1 представлены гистограммы одного репрезентативного эксперимента.
Статистический анализ
Статистический анализ проводили с использованием программы Prism 8.0.1. Нормальность распределения оценивали по критерию Колмогорова–Смирнова. Для анализа статистической значимости различий использовали t-тест для независимых выборок. Взаимосвязь признаков оценивали, рассчитывая коэффициент корреляции Пирсона (r). При множественных сравнениях использовали однофакторный или многофакторный дисперсионный анализ (ANOVA) с последующим тестом Бонферрони. Данные в рисунках представлены в виде среднего значения (Mean) и стандартной ошибки среднего (SEM).
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Экспрессия Tim-3, CD9 на NK периферической крови в I и III триместрах беременности
Установлено, что общее число NK-клеток периферической крови в I триместре беременности не менялось, а в III триместре снижалось по сравнению с небеременными женщинами (рис. 2а). Анализ субпопуляционного состава NK периферической крови показывает, что уменьшение общего числа NK-клеток связано со снижением количества цитотоксических CD16+CD56dimNK в III триместре беременности. Следует отметить, что цитотоксические CD16+CD56dimNK преобладают среди NK периферической крови [9]. При этом количество цитотоксических CD16+CD56dimNK в III триместре снижалось и по сравнению с небеременными и с I триместром. Показано, что среди цитотоксических CD16+CD56dimNK присутствует субпопуляция CD16+CD56–NK (рис. 1а, 2а), которая характеризуется как цитотоксические истощенные NK со сниженной функциональной активностью [5–7]. Процент CD16+CD56–NK достоверно не изменялся по триместрам беременности (рис. 2а). Количество регуляторных CD16–CD56brightNK в I триместре не отличалось от небеременных, но в III триместре их процент увеличивался по сравнению с I триместром (рис. 2а).
Рис. 2.
Оценка (a) общего количества (total) NK (CD3–CD56+) и субпопуляций NK: цитоксической (cytotoxic) (CD16+CD56dimNK), регуляторной (regulatory) (CD16–CD56brightNK) и CD16+CD56–NK. (b) – оценка экспрессия молекул Tim-3, CD9 на цитоксических (cytotoxic) (CD16+CD56dimNK), регуляторных (regulatory) (CD16–CD56bright NK) и CD16+CD56–NK в I и III триместрах беременности. Примечание: На рис. 2а и 2b данные представлены в виде среднего ± стандартной ошибки среднего (Mean ± SEM); I – I триместр беременности, III – III триместр беременности; * p < 0.05, ** p < 0.01 по t-тесту для независимых выборок по отношению к группе небеременных (NP); #p < 0.05 по отношению к I триместру, r – коэффициент корреляции Пирсона.
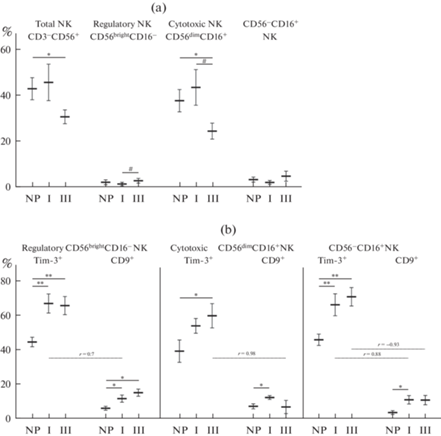
Экспрессия Tim-3 усиливалась в I триместре на регуляторных CD16–CD56brightNK, цитотоксических истощенных CD16+CD56–NK и цитотоксических CD16+CD56dimNK, но на последних недостоверно (рис. 2б), тогда как в III триместре экспрессия Tim-3 была повышена на всех субпопуляциях NK по сравнению с небеременными.
Экспрессия CD9 также менялась по триместрам беременности. Процент регуляторных CD9-позитивных CD16–CD56brightNK был выше и в I, и в III триместрах по сравнению с небеременными (рис. 2б). На цитотоксических CD16+CD56dimNK и CD16+CD56–NK экспрессия CD9 была повышена в I триместре по сравнению с небеременными (рис. 3a, b). В III триместре экспрессия CD9 на цитотоксических CD16+CD56dimNK снижалась по сравнению с I триместром, а на CD16+CD56–NK сохранялась повышенной, хотя и не отличалась достоверно от небеременных.
Рис. 3.
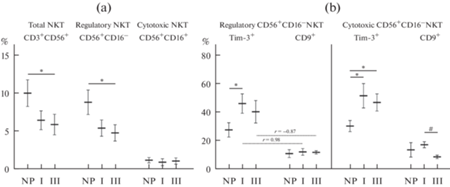
Оценка (a) общего количества (total) NKT (CD3+CD56+) и субпопуляций NKT: цитоксической (cytotoxic) (CD16+CD56+ NKT), регуляторной (regulatory) (CD16–CD56+NKT). (b) – оценка экспрессия молекул Tim-3, CD9 на цитоксических (cytotoxic) (CD16+CD56+NKT), регуляторных (regulatory) (CD16–CD56+NKT) в I и III триместрах беременности. Примечание: На рис. 3а и 3b данные представлены в виде среднего ± стандартной ошибки среднего (Mean ± SEM); I – I триместр беременности, III – III триместр беременности; * p < 0.05, ** p < 0.01 по t-тесту для независимых выборок по отношению к группе небеременных (NP); #p < 0.05 по отношению к I триместру, r – коэффициент корреляции Пирсона.
По данным корреляционного анализа экспрессия CD9 прямо коррелировала с присутствием Tim-3 на регуляторных CD16–CD56brightNK (r = 0.7; p < 0.05) и цитотоксических истощенных CD16+CD56–NK (r = 0.88; p < 0.05) у беременных в I триместре. Выявленная корреляция свидетельствует о возможной взаимосвязи экспрессии этих молекул для субпопуляций NK с низкой цитотоксической активностью. А в III триместре экспрессия CD9 имела обратную зависимость с присутствием Tim-3 на цитотоксических CD16+CD56dimNK (r = –0.98; p < 0.05) и цитотоксических истощенных CD16+CD56–NK (r = –0.93; p < 0.05), что, по-видимому, связано с ограничением миграционной активности Tim-3+ цитотоксических NK.
Таким образом, полученные результаты свидетельствуют о том, что в ранние сроки беременности увеличивается экспрессия молекул Tim-3, CD9 на цитотоксических и регуляторных NK периферической крови, тогда как их общее количество и соотношение не изменяется. В поздние сроки беременности общее количество NK снижается за счет уменьшения числа цитотоксических клеток, а процент регуляторных NK увеличивается, как и экспрессия CD9 на них. Экспрессия Tim-3 остается повышенной и на цитотоксических, и на регуляторных NK.
Экспрессия Tim-3, CD9 на NKT периферической крови в I и III триместрах беременности
Общее количество CD56+NKT в I триместре не отличалось от небеременных, а в III триместре процент CD56+NKT достоверно снижался (рис. 3а). Число регуляторных CD16–CD56+NKT изменялось аналогично общему количеству NKT (рис. 3а). Следует отметить, что в отличие от NK, в популяции NKT периферической крови преобладают регуляторные CD16–CD56+NKT. Количество цитотоксических CD16+CD56+NKT было сравнимо с небеременными и в I, и в III триместрах беременности (рис. 3а).
Экспрессия Tim-3 увеличивалась в I триместре на цитотоксических CD16+CD56+NKT и на регуляторных CD16–CD56+NKT, а в III триместре беременности только на цитотоксических CD16–CD56+NKT (рис. 3b). Экспрессия CD9 как на регуляторных CD16–CD56+NKT, так и на цитотоксических CD16+CD56+NKT не отличалась от небеременных, но в III триместре она снижалась на цитотоксических CD16+CD56+NKT по сравнению с I триместром (рис. 3b). При этом в I триместре беременности экспрессия CD9 прямо коррелировала с количеством Tim-3 на регуляторных CD16–CD56+NKT (r = 0.98; p < 0.05), однако в III триместре, напротив, была выявлена обратная зависимость (r = –0.87; p < 0.05).
Таким образом, в I триместре беременности количество и соотношение разных субпопуляций NKT не изменялись, а экспрессия Tim-3 усиливалась на всех субпопуляциях NKT. В III триместре беременности общее количество NKТ уменьшалось за счет снижения процента регуляторных NKТ, а процент цитотоксических NKТ не изменялся, но экспрессия Tim-3 на них увеличивалась.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
При физиологически протекающей беременности количество и функциональная активность NK и NKT периферической крови существенно меняются, что обусловлено изменением гормонально-цитокинового фона и необходимо для изменения иммунореактивности организма матери c целью сохранения и развития полуаллогенного плода [1, 25, 28]. В матке нарастает количество регуляторных NK и NKT, которые являются ведущими эффекторами иммунной толерантности в зоне фето-плацентарного контакта и выполняют фетотрофическую функцию, модулируют рост спиральных артерий [1, 25, 28]. Децидуальные NK и NKT характеризуются высокой экспрессией молекул Tim-3, CD9, которые играют критическую роль в регуляции их функций [12–19]. Tim-3-сигналинг на NK угнетает их дегрануляцию, цитотоксичность, моделирует продукцию цитокинов [14, 41]. Экспрессия CD9 необходима для взаимодействия с клетками эндотелия и трансэндотелиальной миграции, но также ограничивает дегрануляцию и влияет на продукцию цитокинов [19]. В поздние сроки физиологически протекающей беременности количество децидуальных NK и NKT снижается, а изменения иммунореактивности организма матери связаны с подготовкой к родам [42]. Учитывая критическую роль Tim-3 и CD9 в регуляции функций NK и NKT, а также возможность пополнения децидуальной популяции лимфоцитов клетками периферической крови, важно изучить экспрессию Tim-3, CD9 на цитотоксических и регуляторных периферических NK и NKT в разные сроки беременности.
В проведенном исследовании установлено, что в I триместре физиологически протекающей беременности общее количество и соотношение регуляторных и цитотоксических NK и NKT в периферической крови не менялось. Но экспрессия Tim-3 увеличивалась на регуляторных и цитотоксических NKT, регуляторных CD16–CD56brightNK и цитотоксических истощенных CD16+CD56–NK, кроме цитотоксических CD16+CD56dimNK. Однако процент Tim-3+CD16+CD56dimNK также увеличивался в I триместре, хотя и недостоверно. Повышение экспрессии Tim-3 на всех изученных субпопуляциях NK и NKT в I триместре согласуется с угнетением их цитотоксической активности и изменением спектра продуцируемых цитокинов (снижение выработки IFN-gamma, увеличение секреции IL-4), что характерно для ранних сроков беременности [28–30]. Сходные закономерности экспрессии Tim-3 на периферических CD56+NK описаны в ранние сроки беременности и другими авторами [14, 41]. Следует отметить, что именно регуляторная субпопуляция NKT периферической крови способна к быстрой и массивной продукции поляризующих цитокинов, направляющих развитие иммунного ответа по клеточноопосредованному или гуморальному типу [28–30]. Можно полагать, что увеличение экспрессии Tim-3 на них является одним из потенциальных механизмов, регулирующих продукцию IFN-gamma в ранние сроки беременности, хотя данные механизмы для NKT не исследованы [28–30].
В нашей работе показано, что экспрессия CD9 в ранние сроки беременности повышалась на всех типах NK и не менялась на NKT. Увеличение экспрессии CD9 на регуляторных NK находилось в прямой зависимости от присутствия Tim-3, что, по-видимому, раскрывает один из потенциальных механизмов, способствующих миграции периферических CD16–CD56brightNK с низкой цитотоксической активностью в матку на ранних сроках беременности. Децидуальные NK характеризуются коэкспрессией Tim-3 и CD9 [20]. Увеличение экспрессии CD9 в сочетании с Tim-3 на цитотоксических NK, по-видимому, в большей мере ассоциировано с угнетением их цитотоксической активности и, возможно, миграцией во вторичные лимфоидные органы [43]. Отсутствие изменений в экспрессии CD9 на NKT в ранние сроки беременности связано, по-видимому, с их ограниченной способностью к миграции. Однако выявленная прямая корреляция между присутствием CD9 и Tim-3 на регуляторных NKT свидетельствует о взаимосвязи экспрессии этих молекул на клетках с ограниченной цитотоксичностью.
Таким образом, можно полагать, что увеличение экспрессии CD9 и Tim-3 на NK-клетках и Tim-3 на NKT периферической крови в I триместре физиологически протекающей беременности играет важную в регуляции их функциональная активности, а именно является одним из механизмов снижения их цитотоксичности, модулирует продукцию цитокинов, определяет миграцию регуляторных CD56brightCD16–NK в матку [14, 41].
В III триместре физиологически протекающей беременности общее количество NK и NKТ снижалось, причем для популяции NK за счет уменьшения числа цитотоксических CD56dimCD16+NK, а среди NKТ снижался процент регуляторных CD56+CD16+NKT. Количество же регуляторных CD56brightCD16–NK, напротив, увеличивалось. Экспрессия Tim-3 усиливалась на всех субпопуляциях NK и цитотоксических NKТ, а CD9 повышалась только на регуляторных NK.
Снижение количества цитотоксических CD56dimCD16+NK при увеличении экспрессия Tim-3 на них, а также на цитотоксических NKТ в поздние сроки беременности, по-видимому, является одним из механизмов системного угнетения цитотоксической активности клеток крови при беременности [8, 44, 45] и может быть обусловлено действием гормонов, продуцируемых плацентой. Так, прогестерон, концентрация которого нарастает к концу беременности, усиливает апоптоз NK‑клеток [46]. Необходимо отметить, что цитотоксические NK обладают наибольшей экспрессией рецепторов к прогеcтерону в отличие от регуляторных [41, 46]. Можно полагать, что экспрессия Tim-3 на NK и NKT периферической крови усиливается под влиянием прогестерона, концентрация которого нарастает к концу беременности, поскольку в ряде экспериментальных работ выявлена зависимость экспрессии Tim-3 на CD56+NK и CD56+NKT периферической крови от эффектов прогестерона [34]. Экспрессия Tim-3 повышает чувствительность к апоптозу, что также может объяснять снижение количества NK и NKT периферической крови в III триместре беременности [16]. Однако и другие гормоны, продуцируемые плацентой, а также цитокины влияют на функциональную активность NK и NKT периферической крови. Показана прямая зависимость присутствия Tim-3 на CD56+NK от увеличения концентрации TGF-1beta [14, 41]. Другими авторами установлено, что белки беременности, продуцируемые трофобластом, модулируют экспрессию Tim-3 и CD9 на периферических NK [20]. В наших предыдущих исследованиях показано, что инкубация NK периферической крови человека in vitro c гормонами, продуцируемыми плацентой (эстрадиол, эстриол, прогестерон, хорионический гонадотропин, лептин, грелин, кисспептин), в концентрациях, характерных для разных триместров беременности, оказывала значимые модулирующие эффекты на экспрессию CD16, молекул клеточной адгезии, продукцию перфорина и гранзима, цитокинов, а также регуляторных микроРНК [46–48]. Экспрессия CD9 на цитотоксических NK в поздние сроки не отличалась от небеременных и имела обратную зависимость с присутствием Tim-3, что, по-видимому, связано с ограничением миграционной активности Tim-3+ цитотоксических NK.
Увеличение количества регуляторных NК также может быть обусловлено трансформирующим действием гормонов беременности и иммуносупрессорных цитокинов (TGF-1beta, IL-10), способствующих приобретению децидуально-подобного фенотипа NK-клетками [47–49]. Повышенная экспрессия Tim-3 и CD9 сохранялась на регуляторных NK периферической крови в поздние сроки беременности, что, по-видимому, в большей степени ассоциировано с ограничением дегрануляции и регуляцией продукцией цитокинов, чем с миграцией в матку [50]. Так, в единичных работах показано, что секреция IL-2 была снижена у Tim-3+CD56dimNK по сравнению с Tim-3–CD56dimNK, тогда как Tim-3+CD56brightNK продуцировали больше INF-gamma, чем Tim-3–CD56brightNK [41]. В III триместре экспрессия CD9 на всех исследуемых субпопуляциях NK (кроме регуляторных) и NKT не отличалась от небеременных, что, по-видимому, характеризует уменьшение их миграционной активности и согласуется с данными литературы о том, что максимальное количество децидуальных NK и NKT регистрируется в I триместре беременности, а далее их количество снижается [50].
Цитотоксические истощенные CD16+CD56–NK присутствуют в периферичеcкой крови небеременных, и их процент достоверно не менялся ни в I, ни в III триместрах физиологически протекающей беременности. Экспрессия Tim-3 на них была повышена как в I, так и в III триместрах беременности, а CD9 только в ранние сроки. Функции CD16+CD56–NK при физиологической беременности требуют дальнейшего изучения.
Снижение общего количества периферических NKТ в III триместре беременности происходит за счет уменьшения числа регуляторных NKT, которые преобладают над цитотоксическими, процент которых не меняется. Уменьшение числа регуляторных NKT может объясняться стероид-зависимым угнетением продуктивной функции тимуса, где проходит дифференцировка NKТ, следствием чего является характерная для беременности лимфопения [51]. Динамика субпопуляций регуляторных CD56+CD16–NKТ и цитотоксических CD56+CD16+NKT по триместрам физиологической беременности не изучена. В единичных работах описано изменение количества активированных CD8+CD56+NKT в периферической крови в III триместре беременности [39] и снижение общего количества CD56+NKT [44]. Повышенная экспрессия Tim-3 сохранялась на цитотоксических CD56+CD16+NKT, процент которых не изменялся, что, по-видимому, связано с ограничением их цитотоксической активности.
Таким образом, экспрессия молекул Tim-3 и CD9 на разных субпопуляциях NK и NKT менялась по триместрам, что играет важную роль в регуляции их фенотипа и функций при беременности. Следует отметить, что экспрессия CD9 и Tim-3 характеризует приобретение децидуально-подобного проангиогенного фенотипа NK клетками, свойственного и для NK в опухолевом микроокружении, где ведущую роль в трансформации фенотипа играют иммуносупрессорные цитокины TGF-beta и IL-10 [43, 52, 53].
В целом, можно заключить, что экспрессия изученных молекул на регуляторных и цитотоксических популяциях NK и NKT периферической крови меняется по триместрам беременности, что дополняет наше понимание механизмов регуляции фенотипа и функций NK и NKT периферической крови. Модуляция экспрессии исследованных молекул на NK и NKT периферической крови представляет один из потенциальных терапевтических подходов в регуляции функций NK и NKT для повышения эффективности вспомогательных репродуктивных технологий, а при онкологических заболеваниях необходима для восстановления цитотоксической функции клеток.
Список литературы
Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A (2008) The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol 77(1): 14–22. https://doi.org/10.1016/j.jri.2007.04.007
Shojaei Z, Jafarpour R, Mehdizadeh S, Bayatipoor H, Pashangzadeh S, Motallebnezhad M (2022) Functional prominence of natural killer cells and natural killer T cells in pregnancy and infertility: A comprehensive review and update. Pathol Res Pract 238: 154062. https://doi.org/10.1016/j.prp.2022.154062
Di Santo JP (2008) Functionally distinct NK-cell subsets: developmental origins and biological implications. Eur J Immunol 38(11): 2948–2951. https://doi.org/10.1002/eji.200838830
Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, Faas MM (2002) Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril 77(5): 1032–1037. https://doi.org/10.1016/s0015-0282(02)02976-x
Wijaya RS, Read SA, Schibeci S, Han S, Azardaryany MK, van der Poorten D, Lin R, Yuen L, Lam V, Douglas MW, George J, Ahlenstiel G. (2021). Expansion of dysfunctional CD56–CD16+ NK cells in chronic hepatitis B patients. Liver Int: Offic J Int Associat Study Liver, 41(5): 969–981. https://doi.org/10.1111/liv.14784
Orrantia A, Terrén I, Izquierdo-Lafuente A, Alonso-Cabrera JA, Sandá V, Vitallé J, Moreno S, Tasias M, Uranga A, González C, Mateos JJ, García-Ruiz JC, Zenarruzabeitia O, Borrego F (2020) A NKp80-Based Identification Strategy Reveals that CD56neg NK Cells Are Not Completely Dysfunctional in Health and Disease. Science 23(7): 101298. https://doi.org/10.1016/j.isci.2020.101298
Cocker ATH, Liu F, Djaoud Z, Guethlein LA, Parham P (2022) CD56-negative NK cells: Frequency in peripheral blood, expansion during HIV-1 infection, functional capacity and KIR expression. Front Immunol 13: 992723. https://doi.org/10.3389/fimmu.2022.992723
Whettlock EM, Woon EV, Cuff AO, Browne B, Johnson MR, Male V (2022) Dynamic Changes in Uterine NK Cell Subset Frequency and Function Over the Menstrual Cycle and Pregnancy. Front Immunol 13: 880438. https://doi.org/10.3389/fimmu.2022.880438
Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL (2003) Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 198(8): 1201–1212. https://doi.org/10.1084/jem.20030305
Михайлова ВА, Белякова КЛ, Сельков СА, Соколов ДИ (2017) Особенности дифференцировки NK-клеток: CD56dim и CD56bright NK-клетки во время и вне беременности. Мед иммунол 19(1): 19–26. [Mikhailova VA, Belyakova KL, Selkov SA, Sokolov DI (2017) Features of NK cell differentiation: CD56dim and CD56bright NK cells during and outside pregnancy. Med Immunol 19(1): 19–26. (In Russ)]. https://doi.org/10.15789/1563-0625-2017-1-19-26
Sotnikova N, Voronin D, Antsiferova Y, Bukina E (2014) Interaction of decidual CD56+ NK with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation. Scand J Immunol 80(3): 198–208. https://doi.org/10.1111/sji.12196
Du X, Zhu H, Jiao D, Nian Z, Zhang J, Zhou Y, Zheng X, Tong X, Wei H, Fu B (2022) Human-induced CD49a+NK Cells promote fetal growth. Front Immunol 13: 821542. https://doi.org/10.3389/fimmu.2022.821542
Khademi M, Illés Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallström ET (2004) Cell Ig-and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol 172(11): 7169–7176. https://doi.org/10.4049/jimmunol.172.11.7169
Sun J, Yang M, Ban Y, Gao W, Song B, Wang Y, Zhang Y, Shao Q, Kong B, Qu X (2016) Tim-3 Is upregulated in NK cells during early pregnancy and inhibits NK cytotoxicity toward trophoblast in galectin-9 dependent pathway. PloS One 11(1): e0147186. https://doi.org/10.1371/journal.pone.0147186
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK (2005)The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6(12): 1245–1252. https://doi.org/10.1038/ni1271
Hou H, Liu W, Wu S, Lu Y, Peng J, Zhu Y, Lu Y, Wang F, Sun Z (2014) Tim-3 negatively mediates natural killer cell function in LPS-induced endotoxic shock. PLoS One 9(10): e110585. https://doi.org/10.1371/journal.pone.0110585
Meggyes M, Lajko A, Palkovics T, Totsimon A, Illes Z, Szereday L, Miko E (2015). Feto-maternal immune regulation by TIM-3/galectin-9 pathway and PD-1 molecule in mice at day 14.5 of pregnancy. Placenta 36(10): 1153–1160. https://doi.org/10.1016/j.placenta.2015.07.124
Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, Sukhatme VP, Karumanchi SA, Kopcow HD (2013). Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol (Baltimore, Md: 1950), 190(8): 3939–3948. https://doi.org/10.4049/jimmunol.1202582
Reyes R, Cardeñes B, Machado-Pineda Y, Cabañas C (2018) Tetraspanin CD9: A key regulator of cell adhesion in the immune system. Front Immunol 9: 863. https://doi.org/10.3389/fimmu.2018.00863
Lee CL, Vijayan M, Wang X, Lam KKW, Koistinen H, Seppala M, Li RHW, Ng EHY, Yeung WSB, Chiu PCN (2019) Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype. Hum Reprod 34(4): 689–701. https://doi.org/10.1093/humrep/dey378
Орлова ЕГ, Логинова ОА, Горбунова ОЛ, Каримова НВ, Ширшев СВ (2022) Особенности экспрессии молекул Tim-3, CD9, CD49a лимфоцитами периферической крови при физиологической беременности. [Электронный ресурс] Вестн уральской мед акад науки 19(5): 461–473. [Orlova EG, Loginova ОА, Gorbunova ОL, Karimova NV, Shirshev SV (2022) Features of TIM-3, CD9, CD49a molecule expressions by peripheral blood lymphocytes during physiological pregnancy. [Online] Vestn Ural Med Akad Nauki 19(5): 461–473. (In Russ)]. https://doi.org/10.22138/2500-0918-2022-19-5-461-473
Moore T, Dveksler GS (2014) Pregnancy-specific glycoproteins: complex gene families regulating maternal-fetal interactions. Int J Dev Biol 58(2–4): 273–280. https://doi.org/10.1387/ijdb.130329gd
Kronenberg M, Gapin L (2002) The unconventional lifestyle of NKT cells. Nat Rev Immunol 2: 557–568. https://doi.org/dx.doi.org/10.1038/nri854
Vitelli-Avelar DM, Sathler-Avelar R, Dias JC, Pascoal VP, Teixeira-Carvalho A, Lage PS, Elói-Santos SM, Corrêa-Oliveira R, Martins-Filho OA (2005) Chagasic patients with indeterminate clinical form of the disease have high frequencies of circulating CD3+CD16–CD56+ natural killer T cells and CD4+CD25 high regulatory T lymphocytes. Scand J Immunol 62(3): 297–308. https://doi.org/10.1111/j.1365-3083.2005.01668.x
Yuan J, Li J, Huang SY, Sun X (2015) Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. J Reprod Immunol 110: 81–88. https://doi.org/10.1016/j.jri.2015.05.001
Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L (2004) NKT cells: what’s in a name? Nature Rev Immunol 4(3): 231–237. https://doi.org/10.1038/nri1309
Borzychowski AM, Croy BA, Chan WL, Redman CWG, Sargent IL (2005) Changes in systemic type 1 and type 2 immunity in normal pregnancy and preeclampsia may be mediated by natural killer cells. Eur J Immunol 35: 3054–3063. https://doi.org/10.1002/eji.200425929
Boyson JE, Rybalov B, Koopman LA, Exley M, Balk SP, Racke FK, Schatz F, Masch R, Wilson SB, Strominger JL (2002) CD1d and invariant NKT cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A 9(21): 13741–13746. https://doi.org/10.1073/pnas.162491699
Shi Y, Ling B, Zhou Y, Gao T, Feng D, Xiao M, Feng L (2007) Interferon-gamma expression in natural killer cells and natural killer T cells is suppressed in early pregnancy. Cell Mol Immunol 4(5): 389–394.
Southcombe J, Redman C, Sargent I (2010) Peripheral blood invariant natural killer T cells throughout pregnancy and in preeclamptic women. J Reprod Immunol 87(1–2): 52–59. https://doi.org/10.1016/j.jri.2010.07.003
Van den Heuvel MJ, Peralta CG, Hatta K, Han VK, Clark DA (2007) Decline in number of elevated blood CD3+ CD56+ NKT cells in response to intravenous immunoglobulin treatment correlates with successful pregnancy. Am J Reprod Immunol 58(5): 447–459. https://doi.org/10.1111/j.1600-0897.2007.00529.x
Hosseini S, Shokri F, Pour SA, Khoshnoodi J, Jeddi-Tehrani M, Zarnani AH (2019) Diminished frequency of menstrual and peripheral blood NKT-like cells in patients with unexplained recurrent spontaneous abortion and infertile women. Reprod Sci 26(1): 97–108. https://doi.org/10.1177/1933719118766261
Ito K, Karasawa M, Kawano T, Akasaka T, Koseki H, Akutsu Y, Kondo E, Sekiya S, Sekikawa K, Harada M, Yamashita M, Nakayama T, Taniguchi M (2000) Involvement of decidual Vα14 NKT cells in abortion. Proc Natl Acad Sci U S A 97(2): 740–744. https://doi.org/10.1073/pnas.97.2.740
Lajko A, Meggyes M, Polgar B, Szereday L (2018) The immunological effect of Galectin-9/TIM-3 pathway after low dose Mifepristone treatment in mice at 14.5 day of pregnancy. PLoS One 13(3): e0194870. https://doi.org/10.1371/journal.pone.0194870
Trittel S, Vashist N, Ebensen T, Chambers BJ, Guzmán CA, Riese P (2019) Invariant NKT cell-mediated modulation of ILC1s as a tool for mucosal immune intervention. Front Immunol 10: 1849. https://doi.org/10.3389/fimmu.2019.01849
Ширшев СВ (2009) Иммунология материнско-фетальных взаимодействий. Екатеринбург. УрО РАН. [Shirshev SV (2009) Immunology of maternal-fetal interactions. Ekaterinburg. (In Russ)].
Szekeres-Bartho J (2009) Progesterone-mediated immunomodulation in pregnancy: its relevance to leukocyte immunotherapy of recurrent miscarriage. Immunotherapy (5): 873–882. PMID: https://doi.org/10.2217/imt.09.5420636029
Gutierrez C (1979) Purification of human T and B cells by a discontinuous density gradient of percoll. J Immunol Methods 29(1): 57–63. https://doi.org/10.1016/0022-1759(79)90125-x
De Andrés C, Fernández-Paredes L, Tejera-Alhambra M, Alonso B, Ramos-Medina R, Sánchez-Ramón S (2017) Activation of Blood CD3+CD56+CD8+ T cells during pregnancy and multiple sclerosis. Front Immunol 8: 196. https://doi.org/10.3389/fimmu.2017.00196
Zhou J, Zhao X, Wang Z, Wang J, Sun H, Hu Y (2013) High circulating CD3+CD56+CD16+ natural killer-like T cell levels predict a better IVF treatment outcome. J Reprod Immunol 97(2): 197–203. https://doi.org/10.1016/j.jri.2012.12.006
Meggyes M, Miko E, Polgar B, Bogar B, Farkas B, Illes Z, Szereday L (2014) Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy. PloS One 9(3): e92371. https://doi.org/10.1371/journal.pone.0092371
Williams PJ, Searle RF, Robson SC. Innes BA, Bulmer JN (2009) Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol 82(1): 24–31. https://doi.org/10.1016/j.jri.2009.08.001
Gemelli M, Noonan DM, Carlini V, Pelosi G, Barberis M, Ricotta R, Albini A (2022) Overcoming resistance to checkpoint Inhibitors: natural killer cells in non-small cell lung cancer. Front Oncol 12: 886440. https://doi.org/10.3389/fonc.2022.886440
Meggyes M, Nagy DU, Saad Al Deen I, Parkanyi B, Szereday L (2023) CD8+ and CD8– NKT cells exhibit phenotypic changes during pregnancy. Immunol Invest 52(1): 35–57. https://doi.org/10.1080/08820139.2022.2119863
Mikhailova VA, Kudryavtsev IV, Serebryakova MK, Milyutina YP, Demidova ES, Panina AN, Bazhenov DO, Belikova ME, Selkov SA, Sokolov DI (2020) Trophoblast cell influence on peripheral blood natural killer cell proliferation and phenotype in non-pregnant women and women in early pregnancy. Immunobiology 225(3): 151910. https://doi.org/10.1016/j.imbio.2020.151910
Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, Fainboim L (2008) NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol 180(8): 5746–5753. https://doi.org/10.4049/jimmunol.180.8.5746
Shirshev SV, Nekrasova IV, Gorbunova OL, Orlova EG (2017) Hormonal regulation of NK cell cytotoxic activity. Dokl Biol Sci 472(1): 28–30. https://doi.org/10.1134/S0012496617010021
Shirshev SV, Nekrasova IV, Gorbunova OL, Orlova EG, Maslennikova IL (2017) MicroRNA in hormonal mechanisms of regulation of NK cell function. Dokl Biochem Biophys 474(1): 168–172. https://doi.org/10.1134/S160767291703005X
Mikhailova V, Grebenkina P, Khokhlova E, Davydova A, Salloum Z, Tyshchuk E, Zagainova V, Markova K, Kogan I, Selkov S, Sokolov D (2022) Pro- and Anti-Inflammatory Cytokines in the Context of NK Cell-Trophoblast Interactions. Int J Mol Sci 23(4): 2387. https://doi.org/10.3390/ijms23042387
Van den Heuvel MJ, Chantakru S, Xuemei X, Evans SS, Tekpetey F, Mote PA, Clarke CL, Croy BA (2005) Trafficking of circulating pro-NK cells to the decidualizing uterus: regulatory mechanisms in the mouse and human. Immunol Invest 34(3): 273–293. https://doi.org/10.1081/imm-200064488
Shinomiya N, Tsuru S, Tsugita M, Katsura Y, Takemura T, Roku-tanda M, Nomoto K (1991) Thymic depletion in pregnancy: kinetics ofthymocytes and immunologic capacities of the hosts. J Clin Lab Immunol 34: 11–22.
Montaldo E, Vacca P, Chiossone L, Croxatto D, Loiacono F, Martini S, Ferrero S, Walzer T, Moretta L, Mingari MC (2016) Unique eomes(+) NK cell subsets are present in uterus and decidua during early pregnancy. Front Immunol 6: 646. https://doi.org/10.3389/fimmu.2015.00646
Albini A, Gallazzi M, Palano MT, Carlini V, Ricotta R, Bruno A, Stetler-Stevenson WG, Noonan DM (2021) TIMP1 and TIMP2 downregulate TGFβ induced decidual-like phenotype in natural killer cells. Cancers (Basel) 13(19): 4955. https://doi.org/10.3390/cancers13194955
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова


