Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 9, стр. 1199-1216
Анализ поведения и плотности нейронов в головном мозге мышей B6.Cg-Tg(Prnp-SNCA*A53T)23Mkle/J-модели болезни Паркинсона
И. Н. Рожкова 1, С. В. Окотруб 1, Е. Ю. Брусенцев 1, Т. А. Рахманова 1, 2, Д. А. Лебедева 1, 2, В. С. Козенева 1, 2, Н. В. Хоцкин 1, С. Я. Амстиславский 1, *
1 Институт цитологии и генетики Сибирского отделения Российской академии наук
Новосибирск, Россия
2 Новосибирский национальный исследовательский государственный университет
Новосибирск, Россия
* E-mail: amstis@yandex.ru
Поступила в редакцию 05.06.2023
После доработки 13.07.2023
Принята к публикации 21.07.2023
- EDN: ORGVEX
- DOI: 10.31857/S0869813923090091
Аннотация
Болезнь Паркинсона (БП) является прогрессирующей возрастной нейродегенеративной патологией центральной нервной системы, при которой происходит избирательная потеря дофаминергических нейронов нигростриарного пути и для которой характерно наличие специфических включений (телец Леви) в дофаминовых нейронах. Для изучения механизмов возникновения данной патологии и для поиска возможных путей ее коррекции созданы генетические модели БП на мышах. Трансгенные мыши линии B6.Cg-Tg(Prnp-SNCA*A53T)23Mkle/J (далее в тексте B6.Cg-Tg) имеют мутацию A53T в гене альфа-синуклеина человека и представляют собой модель БП. Целью данной работы было исследование локомоторной активности и уровня тревожности, а также плотности нейронов в головном мозге самцов мышей линии B6.Cg-Tg в возрасте шести месяцев. В качестве контроля были использованы мыши C57BL/6J (дикий тип) того же пола и возраста. Полученные результаты показывают, что для мышей B6.Cg-Tg характерна высокая двигательная активность и низкая тревожность. Наряду с этим у них было обнаружено избирательное снижение плотности нейронов в субвентрикулярной зоне, черной субстанции, а также зонах СА1, СА3, СА4 и гранулярном слое зубчатой извилины гиппокампа. Таким образом, мыши линии B6.Cg-Tg в возрасте шести месяцев лишь частично соответствуют основным патофизиологическим признакам БП, таким как снижение плотности нейронов в черной субстанции, а также в СА1 и СА3 зонах гиппокампа. Однако при этом у них отсутствует брадикинезия и тревожность.
ВВЕДЕНИЕ
Болезнь Паркинсона (БП) является прогрессирующей возрастной нейродегенеративной патологией центральной нервной системы (ЦНС), характеризующейся избирательной потерей дофаминергических нейронов нигростриарного пути и наличием специфических включений (телец Леви) в дофаминовых нейронах [1, 2]. Данное заболевание является вторым по распространенности среди нейродегенеративных расстройств после болезни Альцгеймера и представляет собой разновидность синуклеинопатии [3, 4]. При точечной мутации A53T у людей с БП белок альфа-синуклеин накапливается в различных отделах головного мозга [5, 6]. Обычно альфа-синуклеин распределяется в пресинаптической мембране нейронов, контролируя транспорт синаптических везикул [7]. Избыточное накопление альфа-синуклеина характерно для патологических состояний, а его агрегаты распределяются в телах клеток или нейритах, нарушая функционирование ядра, повреждая синаптические везикулы и большинство органелл нейронов [8]. Такие изменения в ЦНС приводят по мере развития БП к неконтролируемому тремору, ригидности, нарушению двигательной активности и постуральных реакций, когнитивным расстройствам, дисфункции вегетативной нервной системы и нарушению сна [9]. У пациентов с БП выделяют пять стадий развития патологии, при которых наблюдают нарушения в различных структурах головного мозга, в частности в черной субстанции, гиппокампе, полосатом теле и префронтальной коре [10–23]. Черная субстанция является одной из структур головного мозга, которая поражается к третьей стадии БП, что сопровождается чрезмерным накоплением в ее клетках альфа-синуклеина и приводит к двигательным нарушениям [10, 15, 20]. К четвертой стадии БП у пациентов обнаружено накопление альфа-синуклеина в СА2 зоне гиппокампа, важной структуре, отвечающей за обработку пространственной информации, эпизодическую и социальную память [11]. Наряду с этим при БП обнаружены нарушения в СА1 и СА3 зонах гиппокампа [18, 21, 22]. При помощи функциональной магнитно-резонансной томографии было установлено, что в состоянии покоя у людей с БП имеет место снижение спонтанной нейронной активности в префронтальной коре, а также уменьшение серого вещества в данной области, что связывают с нарушением когнитивных функций при БП [13, 14]. Также было показано уменьшение объема хвостатого ядра полосатого тела при БП, что может быть признаком прогрессирования заболевания [16].
Помимо исследований на людях, БП широко изучают на токсических [24–26] и генетических [27–36] моделях БП, созданных на мышах. Генетические модели БП считаются надежными для изучения патогенетических механизмов развития паркинсонизма [27, 28, 37, 38]. Такие модели представлены трансгенными линиями мышей, у которых экспрессируется мутантная форма A30P и/или A53T гена альфа-синуклеина человека [24, 27, 28].
Основными симптомами БП у мышей считают нарушения локомоторной активности, изменение уровня тревожности и когнитивных функций, а также нарушения в различных структурах головного мозга, которые не полностью гомологичны нарушениям, характерным для БП человека [24, 35, 38–41]. Трансгенные гемизиготные мыши линии B6.Cg-Tg(Prnp-SNCA*A53T)23Mkle/J (далее по тексту B6.Cg-Tg) с мутацией A53T в гене альфа-синуклеина человека были созданы в Джексоновской лаборатории (США) [42] и выбраны в качестве модели БП для нашего исследования. Мыши этой линии демонстрируют изменения локомоторной активности в более раннем возрасте, чем мыши с мутацией A30P [28], что создает определенные предпочтения в пользу выбора именно первой модели.
Для изучения двигательного поведения, тревожности, исследовательской активности и когнитивных способностей у мышей с симптомами БП используют тест открытого поля (ОП), приподнятый крестообразный лабиринт (ПКЛ), ротарод, водный тест Морриса, тесты с вращающимся стержнем и тест на распознавание нового объекта [26, 38, 40, 41, 43, 44]. В качестве поведенческих маркеров линий мышей, являющихся моделями БП, чаще всего выбирают двигательную активность и уровень тревожности, хотя результаты этих работ достаточно противоречивы [38–41, 45, 46].
Помимо исследования поведения, у мышей с симптомами БП при помощи гистологических и иммуногистохимических методов изучали развитие нервной системы [26, 30, 44, 47]. Гиппокамп находится в центре внимания этих исследований, поскольку изменения в гиппокампе характерны для БП и некоторых других нейродегенеративных заболеваний [48–50]. В частности, в зонах СА2 и СА3 гиппокампа обнаружены изменения как на генетической модели БП, созданной на мышах B6.C3-Tg(Prnp-SNCA*A53T)83Vle/J (линия B6.C3-Tg), так и у людей при данной патологии [10].
В нашей работе была исследована трансгенная линия B6.Cg-Tg, у которой экспрессируется мутантная форма A53T гена альфа-синуклеина человека, а также имеется ряд биохимических и поведенческих особенностей, характерных для синуклеинопатий [29, 30, 51]. Эта модель воспроизводит основные симптомы синуклеинопатии, включая развивающуюся с возрастом нейродегенерацию, характерную для БП [35, 37]. У мышей B6.Cg-Tg показана обонятельная дисфункция [51]. Однако по координации движений и равновесия в тестах ротарод и подвешивание на проволоке различий с контролем обнаружено не было [51]. Ранее было показано, что мыши B6.Cg-Tg в возрасте пяти–шести месяцев отличаются повышенной локомоторной активностью [52, 53]. До проведения настоящего исследования уровень тревожности у мышей линии B6.Cg-Tg в этом возрасте не оценивали.
В возрасте пяти месяцев у мышей B6.Cg-Tg была обнаружена низкая активность аутофагии в префронтальной коре, стриатуме и черной субстанции по сравнению с контрольными мышами C57BL/6J, а также снижение уровня тирозингидроксилазы в стриатуме, но не в черной субстанции [29]. Кроме того, у B6.Cg-Tg линии выявлено снижение уровня мРНК Cst3 (цистатина С) в стриатуме и миндалевидном теле по сравнению с контролем [30]. По уровню апоптоза нейронов в субвентрикулярной зоне различий между линиями мышей обнаружено не было, однако было установлено снижение нейрогенеза у мышей B6.Cg-Tg [51]. Между тем плотность нейронов в таких важных для развития БП отделах мозга, как префронтальная кора, структуры нигростриарного пути и гиппокамп на данной модели до сих пор не изучали, хотя на сходных моделях было обнаружено уменьшение числа нейронов в различных зонах гиппокампа, а также в черной субстанции [10, 36].
Настоящее исследование направлено 1) на анализ общей и исследовательской активности, а также уровня тревожности; 2) на оценку плотности нейронов в префронтальной коре, CA1, CA2, CA3, СА4 областей и зубчатой извилины гиппокампа, хвостатого ядра полосатого тела и субвентрикулярной зоны, а также черной субстанции у контрольных мышей C57BL/6J (дикий тип) и мышей B6.Cg-Tg (модель болезни Паркинсона).
МЕТОДЫ ИССЛЕДОВАНИЯ
Животные
В эксперименте были исследованы две линии мышей, а именно: семь самцов B6.Cg-Tg(Prnp-SNCA*A53T)23Mkle/J (далее по тексту – B6.Cg-Tg), гемизиготные мыши, моделирующие БП, полученные из Джексоновской лаборатории (США) [42] и 15 самцов контрольной линии C57BL/6J (далее по тексту – C57BL). Животных содержали в SPF-виварии Института цитологии и генетики СО РАН (Новосибирск, Россия) в индивидуально вентилируемых клетках OptiMice (Animal Care, США) размером 34.3 × 29.2 × 15.5 см, при температуре 22–24°C и влажности 40–50%, с инвертированным 12:12-часовым циклом дня–ночи (рассвет в 3 ч утра); в качестве подстила использовали березовую щепу, фракционную для содержания лабораторных животных (ТУ 16.10.23-001-0084157135-2019). Животные имели постоянный доступ к автоклавированному стандартизированному комбикорму для лабораторных крыс и мышей “Дельта Фидс” ЛбК 120 Р-22, ГОСТ 34566-2019 (БиоПро, Россия) и очищенной воде “Северянка” (Экопроект, Россия), обогащенной минеральными добавками.
Анализ поведения
Изучение поведения проводили у семи самцов B6.Cg-Tg и 15 самцов C57BL в возрасте шести месяцев. Общую двигательную активности и тревожность изучали с помощью тестов открытое поле (ОП) и приподнятый крестообразный лабиринт (ПКЛ). Каждого самца помещали в чистую, индивидуально вентилируемую клетку за два дня до тестирования. Для устранения запахов все поверхности камер для тестирования очищали после каждого животного 6%-ным раствором перекиси водорода. Поведение регистрировали с помощью вертикальных видеокамер и анализировали с помощью программно-аппаратного комплекса EthoVisionXT (Noldus, Нидерланды).
Тест открытое поле
Тест OП традиционно используют для оценки поведенческих профилей грызунов [54, 55]. Время проведения испытания с 16:00 до 18:00, что совпадало с началом темного времени суток в комнате содержания животных (16:00), то есть в период наибольшей активности животных. Для проведения теста использовали круглую арену (OpenScience, Россия) диаметром 60 см из белого поливинилхлорида, огороженную стенкой высотой 30 см. Продолжительность теста составляла пять минут. Регистрировали стандартные параметры: 1) пройденное расстояние, 2) время, проведенное в центре, 3) время, проведенное на периферии, 4) число вертикальных стоек и 5) число грумингов. Регистрацию поведения проводили два исследователя вручную.
Тест приподнятый крестообразный лабиринт
Тест ПКЛ используют для оценки уровня тревожности и двигательной активности, часто в сочетании с тестом ОП [55, 56]. Лабиринт, использованный в данном исследовании, состоял из двух открытых (25 × 5/30 × 5.5 см) и двух закрытых (25 × 5 × 30/30 × 5.5 × 14.5 см) рукавов, разделенных центральной ареной (5 × 5 см). Установка располагалась на высоте 50 см над поверхностью пола и освещалась лампой накаливания (40 Вт), расположенной сверху на высоте 130 см. Продолжительность теста составляла пять минут. Регистрировали стандартные параметры: 1) доля времени нахождения в открытых и закрытых рукавах, 2) доля исследованной площади в открытых и закрытых рукавах, 3) число свешиваний с рукавов и 4) число вытягиваний [55, 56].
Интракардиальная перфузия
Перфузию осуществляли через систему кровообращения для фиксации тканей головного мозга, как описано ранее [57]. Сразу после окончания поведенческих тестов мышей наркотизировали, вводя им внутримышечно 75 мкл (на 10 г массы тела) медетомидина гидрохлорида (Медитин, 1 мг/мл; Апи-Сан, Россия) и 60 мкл (на 10 г массы тела) золетила (Virbac, Франция). Далее им вводили через кровеносную систему 30–50 мл фосфатно-солевого буфера (PBS), а затем 4%-ный раствор формалина, приготовленного на PBS. После этого мозг извлекали и помещали в 30%-ный раствор сахарозы, приготовленный на PBS с добавлением 10% формалина, при 4°C для обезвоживания и последующей фиксации в течение следующих 2 недель, пока фиксированный материал не опустится на дно колбы. Образцы мозга погружали в Tissue-Tek O.C.T. (Sakura Finetek, США), затем замораживали и хранили при –70°С.
Приготовление замороженных срезов мозга
По пять животных каждой из исследуемой линии были выбраны случайным образом с целью гистологического анализа мозга. В соответствии с координатами атласа Paxinos и соавт. [58], делали замороженные срезы следующих областей головного мозга: 1) префронтальной коры (ПК) на расстоянии 2.46–2.22 мм от брегмы; 2) хвостатого ядра полосатого тела (ПТ) и субвентрикулярной зоны (subventricular zone – SVZ) на расстоянии 0.74–0.26 мм от брегмы; 3) гиппокампа (области CA1, CA2, CA3, СА4 и зубчатая извилина, dentate gyrus – DG) на расстоянии –1.46 (–1.82) мм от брегмы; 4) черной субстанции (ЧС) на расстоянии –3.20 (–3.60) мм от брегмы. Срезы толщиной 10 мкм готовили при –25°С на криотоме HM550 OP (Thermo Scientific, США) и помещали на предметные стекла Superfrost Plus, Menzel-Glaser (Thermo Fisher Scientific, США).
Иммуногистохимическое окрашивание
Окрашивание образцов проводили по протоколам производителей наборов с небольшими модификациями [57]. Перед процедурой окрашивания срезы обезвоживали с последующей регидратацией в течение пяти минут в PBS. Затем после регидратации в 10 мМ щелочно-цитратного буфера (pH 9) при 95°C на водяной бане (TW-2.02, Elmi, Латвия) в течение 10 мин проводили индуцированную нагреванием демаскировку эпитопов. После этого срезы удаляли из буфера и охлаждали до комнатной температуры. Затем образцы трижды промывали в буфере PBS-Tween: PBS с добавлением 0.1% Tween-20 P9416-100ML (Merck, Германия). После этого в каждую секцию на 30 мин добавляли ProteinBlock (ab64226, Abcam, Великобритания) и удаляли лишнюю жидкость в соответствии с рекомендацией производителя. После этого добавляли 50 мкл первичного антитела и оставляли на ночь при 4°C во влажной темной камере.
Используемые концентрация антитела составляли 1:750 для anti-NeuN (ab177487, Abcam, Великобритания). Затем срезы промывали буфером PBS-Tween, удаляли лишнюю жидкость, добавляли 50 мкл вторичного антитела Goat Anti-Rabbit IgGH & LAF488 (ab150077, Abcam, Великобритания) в концентрации 1 : 650 и оставляли во влажной среде в темной камере на два часа при 4°С. После этого образцы промывали буфером PBS-Tween, удаляли излишки жидкости и монтировали на предметные стекла в среде ProLong, Glass Antifade Mountant (Thermo P36982, Thermo Fisher Scientific, США). После добавления антител срезы обрабатывали в темной камере, защищенной от света.
Анализ плотности нейронов
Анализ плотности меченных антителами нейронов проводили с использованием прямого флуоресцентного микроскопа ZEISS AxioImager 2 (Carl Zeiss, Германия) для ПК (2/3 и 5/6 слоев), ПТ, SVZ, ЧС, а также СА1, СА2, СА3, СА4 (переходная область между СА3 и полиморфным слоем DG) и DG (гранулярного и полиморфного слоев) областей гиппокампа. Для создания поля зрения (5000 мкм2) использовали программу ImageJ. Вычисляли среднее число клеток, меченных антителами в интересующей области с четырех срезов для каждого животного, и рассчитывали среднюю плотность (миллионов клеток, n × 106) в объеме (мм3).
Статистический анализ
Анализ результатов проводили с использованием программы STATISTICA v. 12.0 (StatSoft, Inc., США). Все данные были проверены на нормальность с помощью теста Колмогорова–Смирнова. Поведенческие параметры оценивали с помощью однофакторного дисперсионного анализа с последующим апостериорным сравнением LSD-методом Фишера. Плотность нейронов во всех исследуемых областях головного мозга сравнивали между группами по t-критерию Стьюдента. Данные представлены в виде среднего ± стандартная ошибка среднего (m ± SEM). За уровень значимости принимали р < 0.05.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Тест открытое поле
Результаты исследования мышей в тесте ОП представлены в табл. 1. Однофакторный ANOVA выявил эффект линии [F(1,19) = 22.813; p < 0.001] на параметр “пройденный путь”. Post-hoc анализ при помощи Fisher LSD сравнения показал, что самцы B6.Cg-Tg проходили большую дистанцию (p < 0.001), чем самцы C57BL. Однофакторный ANOVA выявил эффект линии [F(1,19) = 37.59; p < 0.01] на параметр число вертикальных стоек. Post-hoc Fisher LSD показал, что самцы B6.Cg-Tg делали больше стоек (p < 0.01), чем самцы C57BL. Оба параметра свидетельствуют о высокой локомоторной активности мышей B6.Cg-Tg линии.
Таблица 1.
Поведение в тесте открытое поле самцов мышей C57BL и B6.Cg-Tg в возрасте шести месяцев
| Параметры | Группа | F, p | |
|---|---|---|---|
| C57BL | B6.Cg-Tg | ||
| Пройденный путь, см | 2365.3 ± 182.5 | 3956.8 ± 300.1 | F(1,19) = 22.813 p = 0.0001 |
| Время в центре, % от общего | 11.0 ± 2.0 | 7.2 ± 1.4 | F(1,19) = 1.5214 p > 0.05 |
| Время на периферии, % от общего | 89.0 ± 2.0 | 92.8 ± 1.4 | F(1,19) = 1.5214 p > 0.05 |
| Число грумингов | 14.0 ± 2.4 | 22.4 ± 8.6 | F(1,19) = 1.5209 p > 0.05 |
| Число вертикальных стоек | 27.8 ± 3.2 | 45.0 ± 4.8 | F(1,19) = 37.59 p < 0.01 |
Тест приподнятый крестообразный лабиринт
Данные тестирования в тесте ПКЛ представлены в табл. 2. Однофакторный ANOVA выявил эффект линии [F(1,19) = 4.7674; p < 0.05] на пройденный путь. Post-hoc Fisher LSD показал, что самцы B6.Cg-Tg проходили большую дистанцию (p < 0.05), чем самцы C57BL. Однофакторный ANOVA выявил эффект линии [F(1,19) = = 10.822; p < 0.01] на время, проведенное в открытых рукавах. Post-hoc Fisher LSD показал, что самцы B6.Cg-Tg дольше находились в открытых рукавах (p < 0.01), чем самцы C57BL. Однофакторный ANOVA выявил эффект линии [F(1,19) = 9.3112; p < 0.01] на число выглядываний. Post-hoc Fisher LSD показал, что самцы B6.Cg-Tg выглядывали из рукавов лабиринта чаше (p < 0.01), чем самцы C57BL. Однофакторный ANOVA выявил эффект линии [F(1,19) = 7.7549; p < 0.05] на общее время выглядываний. Post-hoc Fisher LSD показал, что самцы B6.Cg-Tg дольше выглядывали из закрытых рукавов (p < 0.05), чем самцы C57BL. Результаты теста ПКЛ свидетельствуют о высокой локомоторной активности и низком уровне тревожности у мышей B6.Cg-Tg линии.
Таблица 2.
Поведение в тесте приподнятый крестообразный лабиринт самцов мышей C57BL и B6.Cg-Tg в возрасте шести месяцев
| Параметры | Группа | F, p | |
|---|---|---|---|
| C57BL | B6.Cg-Tg | ||
| Пройденный путь, см | 1109.9 ± 77.6 | 1403.5 ± 109.7 | F(1,19) = 4.7674 p < 0.05 |
| Время в закрытых рукавах, % от общего | 75.9 ± 3.3 | 67.6 ± 4.7 | F(1,19) = 2.0883 p > 0.05 |
| Время в центре, % от общего | 11.7 ± 2.0 | 11.5 ± 2.8 | F(1,19) = 2.2484 p > 0.05 |
| Время в открытых рукавах, % от общего | 7.4 ± 2.4 | 20.9 ± 3.3 | F(1,19) = 10.822 p < 0.01 |
| Число выглядываний | 8.5 ± 3,0 | 24.3 ± 4.2 | F(1,19) = 9.3112 p < 0.01 |
| Общее время выглядываний, с | 2.8 ± 256 | 15.2 ± 3.6 | F(1,19) = 7.7549 p < 0.05 |
| Число вытягиваний | 11.6 ± 1.3 | 10.4 ± 1.8 | F(1,19) = 0.2698 p > 0.05 |
| Общее время вытягиваний, с | 5.2 ± 1.4 | 9.5 ± 2.0 | F(1,19) = 3.1685 p > 0.05 |
Анализ плотности нейронов
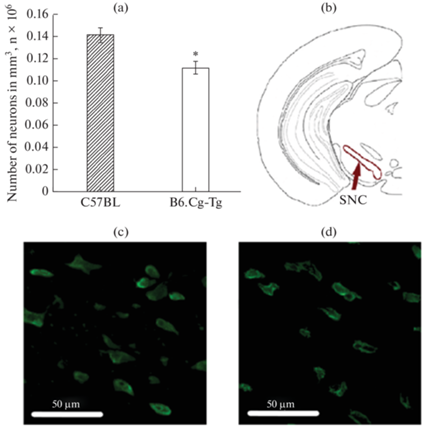
Данные по плотности нейронов в ПК (2/3 и 5/6 слоях), SVZ, ПТ и ЧС представлены на рис. 1–3. Статистический анализ не выявил различий по числу нейронов в ПК (2/3 и 5/6 слоях) и ПТ головного мозга у мышей B6.Cg-Tg и C57BL (рис. 1–2). Однако было обнаружено уменьшение числа нейронов (p < 0.01) в SVZ у мышей B6.Cg-Tg по сравнению с C57BL (0.28 × 106 ± 0.01 × 106 против 0.35 × 106 ± 0.02 × 106) (рис. 2). Кроме того, статистический анализ выявил уменьшение числа нейронов (p < 0.05) в ЧС у мышей B6.Cg-Tg группы по сравнению с контролем (0.11 × 106 ± ± 0.01×106 против 0.14 × 106 ± 0.01 × 106) (рис. 3).
Рис. 1.
Плотность нейронов в префронтальной коре головного мозга (2/3 и 5/6 слои) у самцов мышей B6.Cg-Tg и C57BL, нейроны мечены антителами против NeuN; (a) – число нейронов (в миллионах на мм3); (b) – схематическое обозначение исследуемой области в головном мозге. Микрофотографии срезов мозга данной области: (c–d) – C57BL (2/3 и 5/6 слои соответственно); (e–f) – B6.Cg-Tg (2/3 и 5/6 слои соответственно).
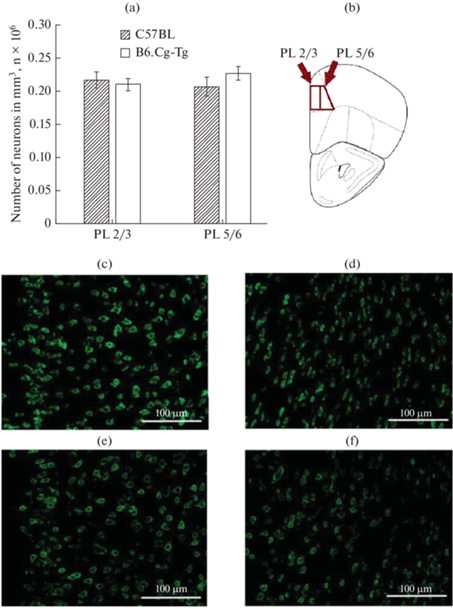
Рис. 2.
Плотность нейронов в субвентрикулярной зоне (subventricular zone – SVZ) и хвостатом ядре полосатого тела (caudoputamen – CP), нейроны мечены антителами против NeuN; (a) – число нейронов (в миллионах на мм3); (b) – схематическое обозначение исследуемых областей в головном мозге. #Микрофотографии срезов мозга данной области: (c) – C57BL; (d) – B6.Cg-Tg. ** p < 0.01.
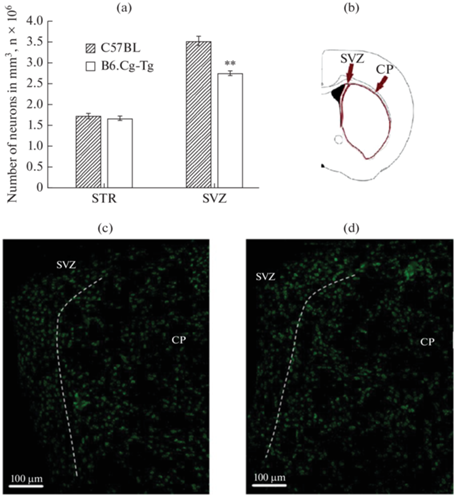
Рис. 3.
Плотность нейронов в черной субстанции (substatia nigra – SNC), нейроны мечены антителами против NeuN; (a) – число нейронов (в миллионах на мм3); (b) – схематическое обозначение исследуемой области в головном мозге. Микрофотографии срезов мозга данной области: (c) – C57BL; (d) – B6.Cg-Tg. * p < 0.05.
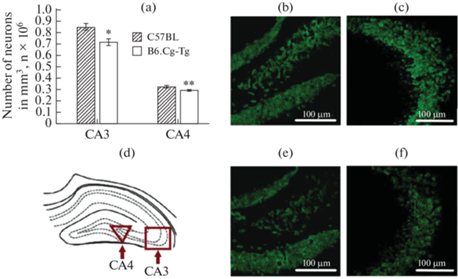
Данные по плотности нейронов в СА1, СА2, СА3, СА4 и DG областях гиппокампа представлены на рис. 4–5. Статистический анализ выявил уменьшение числа нейронов (p < 0.05) в СА1 области у мышей B6.Cg-Tg по сравнению с особями группы C57BL (1.09 × 106 ± 0.06 × 106 против 1.28 × 106 ± 0.03 × 106) (рис. 4). Однако в СА2 области гиппокампа различий по числу нейронов выявлено не было (рис. 4). Также было обнаружено уменьшение числа нейронов в СА3 (p < 0.05) и СА4 (p < 0.01) областях гиппокампа, а также в гранулярном слое DG области (p < 0.01) у мышей B6.Cg-Tg по сравнению с контролем (0.72 × 106 ± 0.03×106 и 0.85 × 106 ± 0.03 × 106 для СА3; 0.29 × 106 ± 0.01 × 106 и 0.33 × 106 ± 0.01 × 106 для СА4; 1.43 × 106 ± 0.04 × 106 и 1.62 × 106 ± 0.02 × 106 для DG) (рис. 5). Однако для полиморфного слоя DG области не было обнаружено различий по числу нейронов у мышей B6.Cg-Tg и C57BL (рис. 4).
Рис. 4.
Плотность нейронов в СА1, СА2 и в гранулярном слое DG областей гиппокампа, нейроны мечены антителами против NeuN; (a) – число нейронов (в миллионах на мм3); (e) – схематическое обозначение исследуемых областей в головном мозге. Микрофотографии срезов мозга данных областей: (b–d) – C57BL (СА1, СА2 и гранулярного слоя DG области гиппокампа соответственно); ( f–h) – B6.Cg-Tg (СА1, СА2 и гранулярного слоя DG области гиппокампа соответственно). * p < 0.05, ** p < 0.01.
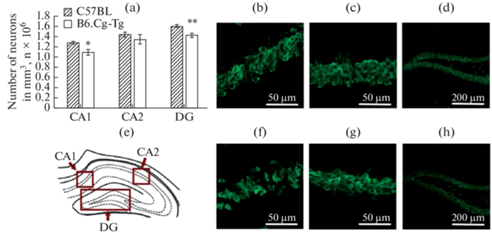
Рис. 5.
Плотность нейронов в СА3 и СА4 областях гиппокампа, нейроны мечены антителами против NeuN; (a) – число нейронов (в миллионах на мм3); (d) – схематическое обозначение исследуемых областей в головном мозге. Микрофотографии срезов мозга данных областей: (b, e) – СА4 область гиппокампа (C57BL и B6.Cg-Tg соответственно); (c, f) – СА3 область гиппокампа (C57BL и B6.Cg-Tg соответственно). * p < 0.05, ** p < 0.01.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
У мышей B6.Cg-Tg была обнаружена высокая локомоторная активность, что было подтверждено в тестах открытое поле и приподнятый крестообразный лабиринт. Наши данные согласуются с результатами, полученными ранее на мышах этой линии [52, 53], а также на других линиях, являющихся моделями БП [46]. У мышей линии hA53T, которая также моделирует БП, двигательная активность также была повышена по сравнению с контролем (диким типом) [46]. В то же время некоторые исследования свидетельствуют, что в определенном возрасте двигательная активность у трансгенных мышей, моделирующих БП, может быть ниже таковой контрольных животных [38, 40, 41]. Так, у трансгенных мышей с мутацией A53T в гене альфа-синуклеина человека наблюдается снижение двигательной активности в возрасте восьми месяцев по сравнению с контролем в тесте ОП [39–41], как и у мышей линий SNCA [59] и A53T-a-Syn [38].
В нашем исследовании впервые изучено поведение мышей B6.Cg-Tg в тесте ПКЛ. Результаты этого теста свидетельствуют о снижении уровня тревожности у мышей B6.Cg-Tg. Ранее на трансгенных мышах TG hA53T SN, которые также моделируют БП, наблюдали снижение уровня тревожности по сравнению с диким типом [45]. В других работах у трансгенных мышей hA53T и A53T-a-Syn, моделирующих БП, наблюдали сложную динамику изменения уровня тревожности с возрастом [38, 46]. Так, в двухмесячном возрасте мыши hA53T проводили меньше времени в открытых рукавах по сравнению с мышами дикого типа, в восьмимесячном – различий по данному параметру с контролем не наблюдали, а в 12-месячном, наоборот, животные данной модели БП проводили больше времени в открытых рукавах по сравнению с контролем [46]. Уровень тревожности у мышей линии A53T-a-Syn, еще одной модели БП, также становился ниже с возрастом [38]. Следует отметить, что на особенности поведения мышей, относящихся к моделям БП, влияет способ получения трансгенной линии, а также их гомозиготность/гетерозиготность по соответствующему гену [27, 45].
Тревога и депрессивно-подобное состояние характерны для БП человека и проявляются еще до появления двигательных симптомов [60]. Механизмы, связанные с изменением поведения в тестах ОП и ПКЛ у мышей B6.Cg-Tg, моделирующих данное заболевание, остаются неясными. Между тем, следует отметить, что нейромедиатор дофамин вовлечен в тревожно-подобное поведение [61]. У трансгенных мышей A53T, моделирующих БП, в возрасте семи месяцев экспрессия дофамина снижена в прилежащем ядре и полосатом теле, что может быть связано с повышенной гиперактивностью, характерной для этой линии [28]. Имеются свидетельства того, что альфа-синуклеин влияет на дофаминовые системы головного мозга через модуляцию его выделения из нейронов [62, 63], влияя на синтез дофамина [64, 65]. Экспрессия A53T может приводить к нарушению регуляции синтеза дофамина у трансгенных мышей, приводя к гиперактивности по механизму, который наблюдают у животных с нокаутом дофамина [66, 67]. Исходя из полученных в нашей работе результатов можно сделать вывод о том, что мыши B6.Cg-Tg в возрасте шести месяцев имеют изменения в поведении, в частности повышение двигательной активности и снижение тревожности, что нехарактерно для БП человека. Однако можно предположить, что с возрастом поведение может поменяться, как уже было показано для некоторых линий мышей с синуклеопатией.
В настоящей работе у мышей B6.Cg-Tg не было обнаружено изменения плотности нейронов в префронтальной коре, полосатом теле, а также в СА2 зоне и полиморфном слое DG области гиппокампа по сравнению с диким типом. Однако в субвентрикулярной зоне, черной субстанции, а также СА1, СА3, СА4 зонах и гранулярном слое DG областей гиппокампа у мышей B6.Cg-Tg число нейронов было существенно ниже по сравнению с контрольной линией. У пациентов с БП также обнаруживают нарушения в гиппокампе, в частности в СА1, СА2 и СА3 зонах [10–12, 21–23]. Роль CA1 в когнитивных нарушениях при БП была подтверждена аутопсией [21] и при помощи магнитно-резонансной томографии [22]. Было показано снижение объемов СА2–СА3 зон и эпизодической памяти при БП [23]. Снижение числа нейронов в SVZ и ЧС головного мозга у мышей B6.Cg-Tg может быть связано со снижением уровня нейрогенеза в субвентрикулярной зоне, что было подтверждено экспериментально на данной линии [51], либо с обнаруженным у них низким уровнем аутофагии в черной субстанции [29]. Деградация нейронов в данной области головного мозга было отмечено также у мышей линии Pitx3-A53Tα-Syn, являющейся трансгенной моделью БП [36], и характерно для болезни Паркинсона у людей [15, 20].
Наблюдаемое нами снижение общей плотности нейронов в СА3 области гиппокампа может быть связано с повышенной экспрессией альфа-синуклеина и уменьшением числа соматостатин-позитивных нейронов в данной области, что было показано на мышах трансгенной линии B6.C3-Tg [10], которая очень похожа на изучаемую нами модель БП. Снижение плотности кальретинин- и кальбиндин-позитивных нейронов обнаружено у мышей B6.C3-Tg в СА1 зоне, а также кальретинин-позитивных нейронов в гранулярном слое DG области гиппокампа [10]. Именно в этих областях нами было обнаружено снижение общего числа нейронов у мышей линии B6.Cg-Tg.
Особый интерес представляет СА4 область гиппокампа, в которой у мышей был обнаружен уникальный набор нейронов, отличный от соседних областей гиппокампа [68]. Данная зона представляет собой структуру между СА3 и DG областями и состоит преимущественно из пирамидных клеток, играет важную роль в формировании ассоциативной памяти, за счет опосредованных возбуждающих и тормозящих сигналов от СА3 зоны к гранулярному слою зубчатой извилины [69]. Некоторые исследователи не выделяют СА4 как отдельную область, считая ее частью полиморфного слоя DG [69]. Однако было показано, что область СА4 у мышей отличается плотностью рецепторов от зон СА3 и DG; в частности, в ней наблюдали высокие показатели экспрессии каинатных и AMPA-рецепторов [69]. Кроме того, зона СА4 отличается более низкой плотностью катехоламинергических и бензодиазепиновых, но не ГАМК-А-рецепторов по сравнению с другими областями гиппокампа [69]. Эти различия, а также функциональная специфика позволяет выделить CA4 в самостоятельную область гиппокампа у мышей [69]. Ранее анализ плотности нейронов в СА4 области гиппокампа у мышей, моделирующих БП, не проводили. В нашем исследовании впервые показано уменьшение плотности нейронов в СА4 области гиппокампа у мышей B6.Cg-Tg.
Гиппокамп является структурой мозга, имеющей ключевое значение для разных типов памяти [70]. На поздних этапах БП для нейронов этой структуры характерно накопление альфа-синуклеина, что сопровождается деменцией [71, 72]. Обнаруженное в настоящем исследовании изменение плотности нейронов в областях СА1, СА3, СА4 и гранулярном слое зубчатой извилины гиппокампа можно рассматривать как один из биомаркеров данной патологии, что показано как в нашем исследовании на модели B6.Cg-Tg, так и частично обнаружено другими авторами на сходной генетической модели БП, созданной на мышах [10].
ЗАКЛЮЧЕНИЕ
В данной работе у самцов мышей B6.Cg-Tg линии было установлено повышение локомоторной активности, снижение тревожности и уменьшение плотности нейронов в субвентрикулярной зоне, черной субстанции, а также в СА1, СА3, СА4 и гранулярном слое зубчатой извилины гиппокампа, что можно рассматривать как биомаркеры синуклеопатии. Дальнейшие исследования будут сосредоточены на попытках направленного воздействия в ходе пренатального развития, чтобы, в конечном итоге, обнаружить новые мишени для профилактики этой патологии.
Список литературы
Yun JW, Ahn JB, Kang BC (2015) Modeling Parkinson’s disease in the common marmoset (Callithrix jacchus): overview of models, methods, and animal care. Lab Anim Res 31: 155–165. https://doi.org/10.5625/lar.2015.31.4.155
Goswami P, Joshi N, Singh S (2017) Neurodegenerative signaling factors and mechanisms in Parkinson’s pathology. Toxicol In Vitro 43: 104–112. https://doi.org/10.1016/j.tiv.2017.06.008
Crotty GF, Schwarzschild MA (2022) What to test in parkinson disease prevention trials? Repurposed, low-risk, and gene-targeted drugs. Neurology 99: 34–41. https://doi.org/10.1212/WNL.0000000000200238
Crotty GF, Ascherio A, Schwarzschild MA (2017) Targeting urate to reduce oxidative stress in Parkinson disease. Exp Neurol 298: 210–224. https://doi.org/10.1016/j.expneurol.2017.06.017
Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 110: 517–536. https://doi.org/10.1007/s00702-002-0808-2
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047. https://doi.org/10.1126/science.276.5321.2045
Maroteaux L, Campanelli JT, Scheller RH (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8: 2804–2815. https://doi.org/10.1523/JNEUROSCI.08-08-02804.1988
Wong YC, Krainc D (2017) α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23: 1–13. https://doi.org/10.1038/nm.4269
Albers JA, Chand P, Anch AM (2017) Multifactorial sleep disturbance in Parkinson’s disease. Sleep Med 35: 41–48. https://doi.org/10.1016/j.sleep.2017.03.026
Flores-Cuadrado A, Ubeda-Banon I, Saiz-Sanchez D, de la Rosa-Prieto C, Martinez-Marcos A (2016) Hippocampal α-synuclein and interneurons in Parkinson’s disease: Data from human and mouse models. Mov Disord 31: 979–988. https://doi.org/10.1002/mds.26586
Villar-Conde S, Astillero-Lopez V, Gonzalez-Rodriguez M, Villanueva-Anguita P, Saiz-Sanchez D, Martinez-Marcos A, Flores-Cuadrado A, Ubeda-Banon I (2021) The human hippocampus in Parkinson’s disease: An Integrative Stereological and Proteomic Study. J Parkinsons Dis 11: 1345–1365. https://doi.org/10.3233/JPD-202465
Prakash KG, Bannur BM, Chavan MD, Saniya K, Sailesh KS, Rajagopalan A (2016) Neuroanatomical changes in Parkinson’s disease in relation to cognition: An update. J Adv Pharm Technol Res 7: 123–126. https://doi.org/10.4103/2231-4040.191416
Kang DZ, Chen FX, Chen FY,Liu Y, Wu G, Yu LH, Lin YX, Lin ZY (2016) Altered regional homogeneity of prefrontal cortex in Parkinson’s disease with mild cognitive impairment. Chin Neurosurg J l2: 10. https://doi.org/10.1186/s41016-016-0028-5
Biundo R, Formento-Dojot P, Facchini S, Vallelunga A, Ghezzo L, Foscolo L, Meneghello F, Antonini A (2011) Brain volume changes in Parkinson’s disease and their relationship with cognitive and behavioural abnormalities. J Neurol Sci 310: 64–69. https://doi.org/10.1016/j.jns.2011.08.001
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 23: 6587–6596. https://doi.org/10.1038/nrdp.2017.13
Lee SH, Kim SS, Tae WS, Lee SY, Choi JW, Koh SB, Kwon DY (2011) Regional volume analysis of the Parkinson disease brain in early disease stage: gray matter, white matter, striatum, and thalamus. AJNR Am J Neuroradiol 32: 682–687. https://doi.org/10.3174/ajnr.A2372
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L (2004) Movement disorder society task force on rating scales for Parkinson’s disease. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19: 1020–1028. https://doi.org/10.1002/mds.20213
Das T, Hwang JJ, Poston KL (2019) Episodic recognition memory and the hippocampus in Parkinson’s disease: A review. Cortex 113: 191–209. https://doi.org/10.1016/j.cortex.2018.11.021
Pagano G, Niccolini F, Politis M (2016) Imaging in Parkinson’s disease. Clin Med (Lond) 16: 371–375. https://doi.org/10.7861/clinmedicine.16-4-371
Bae YJ, Kim JM, Sohn CH, Choi JH, Choi BS, Song YS, Nam Y, Cho SJ, Jeon B, Kim JH (2021) Imaging the substantia nigra in Parkinson disease and other Parkinsonian syndromes. Radiology 300: 260–278. https://doi.org/10.1148/radiol.2021203341
Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, Masliah E, Gage FH (2017) Hippocampal α-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J Neurosci 37: 1675–1684. https://doi.org/10.1523/JNEUROSCI.3047-16.2016
Stav AL, Johansen KK, Auning E, Kalheim LF, Selnes P, Bjørnerud A, Hessen E, Aarsland D, Fladby T (2016) Hippocampal subfield atrophy in relation to cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease: a cross-sectional study. NPJ Parkinsons Dis 2: 15030. https://doi.org/10.1038/npjparkd.2015.30
Foo H, Mak E, Chander RJ, Ng A, Au WL, Sitoh YY, Tan LC, Kandiah N (2016) Associations of hippocampal subfields in the progression of cognitive decline related to Parkinson’s disease. Neuroimage Clin 14: 37–42. https://doi.org/10.1016/j.nicl.2016.12.008
Григорян ГА, Базян АС (2007) Экспериментальные модели болезни Паркинсона на животных. Успехи физиол наук 38: 80–88. [Grigoryan GA, Bazyan AS (2007) The experimental models of Parkinson’s disease in animals. Advanc Physiol Sci 38: 80–88. (In Russ)].
Siani F, Greco R, Levandis G, Ghezzi C, Daviddi F, Demartini C, Vegeto E, Fuzzati-Armentero MT, Blandini F (2017) Influence of estrogen modulation on glia activation in a murine model of Parkinson’s disease. Front Neurosci 11: 306. https://doi.org/10.3389/fnins.2017.00306
Tang H, Gao Y, Zhang Q, Nie K, Zhu R, Gao L, Feng S, Wang L, Zhao J, Huang Z, Zhang Y, Wang L (2017) Chronic cerebral hypoperfusion independently exacerbates cognitive impairment within the pathopoiesis of Parkinson’s disease via microvascular pathologys. Behav Brain Res 333: 286–294. https://doi.org/10.1016/j.bbr.2017.05.061
Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, Sondel J, Kotilinek L, Day J, Schwarzschild MA, Cha JH, Newell K, Miller DW, Ueda K, Young AB, Hyman BT, Ashe KH (2003) Motor dysfunction and gliosis with preserved dopaminergic markers in human alpha-synuclein A30P transgenic mice. Neurobiol Aging 24: 245–258. https://doi.org/10.1016/s0197-4580(02)00091-x
Unger EL, Eve DJ, Perez XA, Reichenbach DK, Xu Y, Lee MK, Andrews AM (2006) Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human alpha-synuclein in mice. Neurobiol Dis 21: 431–443. https://doi.org/10.1016/j.nbd.2005.08.005
Pupyshev AB, Korolenko TA, Akopyan AA, Amstislavskaya TG, Tikhonova MA (2018) Suppression of autophagy in the brain of transgenic mice with overexpression of A53T-mutant α-synuclein as an early event at synucleinopathy progression. Neurosci Lett 672: 140–144. https://doi.org/10.1016/j.neulet.2017.12.001
Korolenko TA, Shintyapina AB, Belichenko VM, Pupyshev AB, Akopyan AA, Fedoseeva LA, Russkikh GS, Vavilin VA, Tenditnik MV, Lin C-L, Amstislavskaya TG, Tikhonova MA (2020) Early Parkinson’s disease-like pathology in a transgenic mouse model involves a decreased Cst3 mRNA expression but not neuroinflammatory response in the brain. Med Univer 3: 66–78. https://doi.org/10.2478/medu-2020-0008
Taguchi T, Ikuno M, Hondo M, Parajuli LK, Taguchi K, Ueda J, Sawamura M, Okuda S, Nakanishi E, Hara J, Uemura N, Hatanaka Y, Ayaki T, Matsuzawa S, Tanaka M, El-Agnaf OMA, Koike M, Yanagisawa M, Uemura MT, Yamakado H, Takahashi R (2020) α-SynucleinBAC transgenic mice exhibit RBD-like behaviour and hyposmia: a prodromal Parkinson’s disease model. Brain 143: 249–265. https://doi.org/10.1093/brain/awz380
Huang Y, Liu Z, Li N, Tian C, Yang H, Huo Y, Li Y, Zhang J, Yu Z (2022) Parkinson’s disease derived exosomes aggravate neuropathology in SNCA*A53T Mice. Ann Neurol 92: 230–245. https://doi.org/10.1002/ana.26421
Karikari AA, McFleder RL, Ribechini E, Blum R, Bruttel V, Knorr S, Gehmeyr M, Volkmann J, Brotchie JM, Ahsan F, Haack B, Monoranu CM, Keber U, Yeghiazaryan R, Pagenstecher A, Heckel T, Bischler T, Wischhusen J, Koprich JB, Lutz MB, Ip CW (2022) Neurodegeneration by α-synuclein-specific T cells in AAV-A53T-α-synuclein Parkinson’s disease mice. Brain Behav Immun 101: 194–210. https://doi.org/10.1016/j.bbi.2022.01.007
Wang Y, Sun Z, Du S, Wei H, Li X, Li X, Shen J, Chen X, Cai Z (2022) The increase of α-synuclein and alterations of dynein in A53T transgenic and aging mouse. J Clin Neurosci 96: 154–162. https://doi.org/10.1016/j.jocn.2021.11.002
Zhang Y, Wu Q, Ren Y, Zhang Y, Feng L (2022) A53T α-synuclein induces neurogenesis impairment and cognitive dysfunction in line M83 transgenic mice and reduces the proliferation of embryonic neural stem cells. Brain Res Bull 182: 118–129. https://doi.org/10.1016/j.brainresbull.2022.02.010
Zheng M, Liu Y, Xiao Z, Jiao L, Lin X (2022) Tau knockout and α-synuclein A53T synergy modulated parvalbumin-positive neurons degeneration staging in substantia nigra pars reticulata of Parkinson’s disease-liked model. Front Aging Neurosci 13: 784665. https://doi.org/10.3389/fnagi.2021.784665
Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson’s disease. Neuron 66: 646–661. https://doi.org/10.1016/j.neuron.2010.04.034
Oaks AW, Frankfurt M, Finkelstein DI, Sidhu A (2013) Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS One 8: e60378. https://doi.org/10.1371/journal.pone.0060378
Liu Y, Yuan YH, Sun JD, Li J, Li ZP, Chen NH (2014) Nigrostriatal dynein changes in A53T alpha-synuclein transgenic mice. F1000Res 3: 68. https://doi.org/10.12688/f1000research.3507.1
Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z, Liu J, Feng L (2019) Caffeic acid reduces A53T α‑synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res 150: 104538. https://doi.org/10.1016/j.phrs.2019.104538
Park HJ, Zhao TT, Kim SH, Lee CK, Hwang BY, Lee KE, Lee MK (2020) Ethanol extract from Gynostemmapentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein. Neural Regen Res 15: 361–368. https://doi.org/10.4103/1673-5374.265557
https://www.jax.org/strain/006823
Mograbi KM, de Castro AC, de Oliveira JA, Sales PJ, Covolan L, Del Bel EA, de Souza AS (2017) Effects of GABAa receptor antagonists on motor behavior in pharmacological Parkinson’s disease model in mice. Physiol Rep 5: e13081. https://doi.org/10.14814/phy2.13081
Langley MR, Ghaisas S, Palanisamy BN, Ay M, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2021) Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson’s disease. Exp Neurol 341: 113716. https://doi.org/10.1016/j.expneurol.2021.113716
George S, van den Buuse M, San Mok S, Masters CL, Li QX, Culvenor JG (2008) Alpha-synuclein transgenic mice exhibit reduced anxiety-like behaviour. Exp Neurol 210: 788–792. https://doi.org/10.1016/j.expneurol.2007.12.017
Graham DR, Sidhu A (2010) Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res 88: 1777–1183. https://doi.org/10.1002/jnr.22331
Oh SH, Lee SC, Kim DY, Kim HN, Shin JY, Ye BS, Lee PH (2017) Mesenchymal stem cells stabilize axonal transports for autophagic clearance of α-synuclein in parkinsonian models. Stem Cells 35: 1934–1947. https://doi.org/10.1002/stem.2650
Lenka A, Ingalhalikar M, Shah A, Saini J, Arumugham SS, Hegde S, George L, Reddy V, Reddy YCJ, Yadav R, Pal PK (2018) Hippocampal subfield atrophy in patients with Parkinson’s disease and psychosis. J Neural Transm (Vienna) 125: 1361–1372. https://doi.org/10.1007/s00702-018-1891-3
DeFlitch L, Gonzalez-Fernandez E, Crawley I, Kang SH (2022) Age and Alzheimer’s disease-related oligodendrocyte changes in hippocampal subregions. Front Cell Neurosci 16: 847097. https://doi.org/10.3389/fncel.2022.847097
Schmitt A, Tatsch L, Vollhardt A, Schneider-Axmann T, Raabe FJ, Roell L, Heinsen H, Hof PR, Falkai P, Schmitz C (2022) Decreased oligodendrocyte number in hippocampal subfield CA4 in schizophrenia: a replication study. Cells 11: 3242.
Seo JH, Kang SW, Kim K, Wi S, Lee JW, Cho SR (2020) Environmental enrichment attenuates oxidative stress and alters detoxifying enzymes in an A53T α-synuclein transgenic mouse model of Parkinson’s disease. Antioxidants (Basel) 9: 928. https://doi.org/10.3390/antiox9100928
Tikhonova MA, Tikhonova NG, Tenditnik MV, Ovsyukova MV, Akopyan AA, Dubrovina NI, Amstislavskaya TG, Khlestkina EK (2020) Effects of grape polyphenols on the life span and neuroinflammatory alterations related to neurodegenerative Parkinson disease-like disturbances in mice. Molecules 25: 5339. https://doi.org/10.3390/molecules25225339
Tikhonova MA, Shoeva OY, Tenditnik MV, Ovsyukova MV, Akopyan AA, Dubrovina NI, Amstislavskaya TG, Khlestkina EK (2020) Evaluating the effects of grain of isogenic wheat lines differing in the content of anthocyanins in mouse models of neurodegenerative disorders. Nutrients 12: 3877. https://doi.org/10.3390/nu12123877
Kulikov VA, Khotskin NV, Nikitin SV, Lankin VS, Kulikov AV, Trapezov OV (2014) Application of 3-D imaging sensor for tracking minipigs in the open field test. J Neurosci Methods 235: 219–225. https://doi.org/10.1016/j.jneumeth.2014.07.012
Khotskin NV, Plyusnina AV, Kulikova EA, Bazhenova EY, Fursenko DV, Sorokin IE, Kolotygin I, Mormede P, Terenina EE, Shevelev OB, Kulikov AV (2019) On association of the lethal yellow (AY) mutation in the agouti gene with the alterations in mouse brain and behavior. Behav Brain Res 359: 446–456. https://doi.org/10.1016/j.bbr.2018.11.013
Ragaeva DS, Tikhonova MA, Petrova OM, Igonina TN, Rozkova IN, Brusentsev EY, Amstislavskaya TG, Amstislavsky SY (2017) Neonatal reflexes and behavior in hypertensive rats of ISIAH strain. Physiol Behav 175: 22–30. https://doi.org/10.1016/j.physbeh.2017.03.026
Рожкова ИН, Окотруб СВ, Брусенцев ЕЮ, Ульданова ЕЕ, Чуйко ЭА, Напримеров ВА, Липина ТВ, Амстиславская ТГ, Амстиславский СЯ (2023) Изменения в социальном предпочтении места и плотность дофаминергических нейронов в вентральном тегментуме у Clsnt2-KO мышей. Вавиловск журн генетики и селекции 27: 177–184. [Rozhkova IN, Okotrub SV, Brusentsev EYu, Uldanova KE, Chuyko EА, Naprimerov VA, Lipina TV, Amstislavskaya TG, Amstislavsky SYa (2023) Alterations in the social-conditioned place preference and density of dopaminergic neurons in the ventral tegmental area in Clsnt2-KO mice. Vavilov J Genet Breed 27: 177–184. (In Russ)]. https://doi.org/10.18699/VJGB-23-14
Paxinos G, Franklin K (2012) Mouse brain in stereotaxic coordinates. 4th ed., Acad Press.
Rothman SM, Griffioen KJ, Vranis N, Ladenheim B, Cong WN, Cadet JL, Haran J, Martin B, Mattson MP (2013) Neuronal expression of familial Parkinson’s disease A53T α-synuclein causes early motor impairment, reduced anxiety and potential sleep disturbances in mice. J Parkinsons Dis 3: 215–229. https://doi.org/10.3233/JPD-120130
Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA (2000) Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord 15: 669–677. https://doi.org/10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5
Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Wetsel WC (2005) Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology 30: 1818–1831. https://doi.org/10.1038/sj.npp.1300724
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lackingalpha-synuclein display functional deficits in the nigrostriatal dopaminesystem. Neuron 25: 239–252. https://doi.org/10.1016/s0896-6273(00)80886-7
Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA (2001) Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci 21: 9549–9560. https://doi.org/10.1523/JNEUROSCI.21-24-09549.2001
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22: 3090–3099. https://doi.org/10.1523/JNEUROSCI.22-08-03090.2002
Lee FJ, Liu F, Pristupa ZB, Niznik HB (2001) Direct binding andfunctional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J 15: 916–926. https://doi.org/10.1096/fj.00-0334com
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612. https://doi.org/10.1038/379606a0
Uhl GR, Vandenbergh DJ, Miner LL (1996) Knockout mice and dirty drugs. Drug addiction. CurrBiol 6: 935–936. https://doi.org/10.1016/s0960-9822(02)00630-9
Lothmann K, Deitersen J, Zilles K, Amunts K, Herold C (2021) New boundaries and dissociation of the mouse hippocampus along the dorsal-ventral axis based on glutamatergic, GABAergic and catecholaminergic receptor densities. Hippocampus 31: 56–78. https://doi.org/10.1002/hipo.23262
Lisman JE, Talamini LM, Raffone A (2005) Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Networks 18: 1191–1201. https://doi.org/10.1016/j.neunet.2005.08.008
Amaral DG, Scharfman HE, Lavenex P (2007) The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163: 3–22. https://doi.org/10.1016/S0079-6123(07)63001-5
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21: 2042–2051. PMID: https://doi.org/10.1002/mds.2106517078043
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9: 13–24. https://doi.org/10.1038/nrneurol.2012.242
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова


