Российский физиологический журнал им. И.М. Сеченова, 2023, T. 109, № 9, стр. 1185-1198
Механизмы антипролиферативного действия стрептококковой аргининдеиминазы в отношении клеток лимфобластной лейкемии линии Jurkat
Э. А. Старикова 1, 2, 3, *, Дж. Т. Маммедова 1, А. Ожиганова 1, Л. А. Бурова 1, И. В. Кудрявцев 1, 2
1 Институт экспериментальной медицины
Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет
им. акад. И.П. Павлова Министерства здравоохранения Российской Федерации
Санкт-Петербург, Россия
3 Институт медицинского образования Национального медицинского исследовательского це-нтра им. В.А. Алмазова Министерства здравоохранения Российской Федерации
Санкт-Петербург, Россия
* E-mail: Starickova@yandex.ru
Поступила в редакцию 23.05.2023
После доработки 12.07.2023
Принята к публикации 21.07.2023
- EDN: ORFKIS
- DOI: 10.31857/S086981392309011X
Аннотация
Стратегия депривации аргинина рассматривается как перспективное направление терапии раковых заболеваний. Целью исследования было изучение влияния аргининдеиминазы пиогенного стрептококка на клетки лимфобластной лейкемии Jurkat. Для этого сравнивали эффекты супернатантов разрушенных стрептококков исходного штамма, экспрессирующего аргининдеиминазу, и его изогенного мутанта с инактивированным геном аргининдеиминазы – arcA. Пролиферацию клеток оценивали в МТТ-тесте. Остальные параметры исследовали с помощью проточной цитометрии. Распределение клеток по фазам клеточного цикла изучали с использованием ДНК-связывающего красителя DAPI и антител против циклина А2. Интенсивность аутофагии оценивали с помощью реагента LysoTracker™ Green DND-26. Для оценки жизнеспособности клетки докрашивали DAPI. Исследования показали, что стрептококковый фермент подавлял пролиферативную активность клеток Jurkat, повышал долю клеток в фазах покоя G0/G1, снижал долю клеток в фазах синтеза S/G2 и усиливал аутофагию без снижения жизнеспособности. Добавка аргинина нивелировала эффекты аргининдеиминазы. Полученные результаты открывают возможность использования аргинин-гидролизующей активности стрептококкового фермента для сочетанной терапии онкологических заболеваний.
ВВЕДЕНИЕ
Аргинин является условно незаменимой аминокислотой, которая вовлечена в жизненно важные физиологические и патологических процессы [1]. Аргинин необходим для эффективного иммунного ответа и регенерации [2, 3]. В условиях стресса и различных катаболических состояниях, включая гемолитические анемии [4–7], астму [8, 9], беременность [10], сепсис, ожоги и травмы [2, 11–13], когда способность эндогенного синтеза аминокислоты не может компенсировать потребности организма, аргинин становится незаменимым.
Метаболизм аргинина играет двойственную роль при опухолевом росте. С одной стороны, дефицит этой аминокислоты вызывает иммуносупрессию и подавление противоопухолевых иммунных реакций [3, 14]. С другой стороны, опухолевые клетки сами нуждаются в аргинине для поддержания высокого уровня пролиферативной активности [3, 15].
Аргинин относится к протеиногенным аминокислотам, поэтому в случае его дефицита останавливается синтез белка [16]. При этом в клетке из-за накопления незаряженных аминоацил-тРНК происходит активация стресс киназы – general control nonderepressible 2 (GCN2) и ее нижележащей мишени – фактора инициации трансляции (eukaryotic translation initiation factor 2A, eIF2A). Когда содержание аргинина истощается, клетки подвергаются остановке клеточного цикла при G0/G1 и пребывают в состоянии покоя до тех пор, пока концентрация аргинина не повысится до нормального уровня [17].
В исследованиях in vitro было установлено, что в опухолевых клетках на ранней стадии аргининового голодания (0–48 ч) происходит инактивация аргинин-зависимого сигнального каскада mechanical target of rapamycin – mTOR [18]. Одним из последствий этого становится усиление киназной активности Unc-51 like autophagy activating kinase – ULK1/2, которая запускает фосфорилирование Autophagy Related 13 – Atg13 и аутофагию [19, 20]. Во время депривации аргинина на поздних стадиях в различных типах раковых клеток (рак поджелудочной железы, острый лимфобластный лейкоз, рак мочевого пузыря и печени) происходит индукция апоптоза [21].
Производные аргинина – оксид азота (nitric oxide, NO), полиамины, нуклеотиды, пролин и глутамат способствуют росту и мутагенезу опухолевых клеток [15]. Роль аргинина при опухолевом росте подтверждают данные о том, что ферменты метаболизма аргинина (орнитиндекарбоксилаза, индуцибельная синтаза оксида азота (inducible nitric oxide synthase, iNOS), аргиназа) и переносчики аминокислот (cationic amino acid transporter (CAT) и Solute Carrier Family 6 Member 14 (SLC6A14)) задействованы в развитии опухоли [18]. Опухолевые клетки нуждаются в аргиназах для лучшей пролиферации и метастазирования. Например, аргиназа 1 в клетках нейробластомы запускает экспрессию сигнальных каскадов AKT и ERK, индуцирующих деление клеток [22]. Активность аргиназы II в опухолях щитовидной железы связана с повышенной экспрессией пролиферативных маркеров Ki-67 и PCNA [23]. Экспрессия аргиназы II на клетках меланомы способствует пролиферативному и миграторному фенотипу клеток с высоким уровнем молекулы адгезии ICAM-1 [24]. Экспрессия iNOS при раке мочевого пузыря человека считается плохим прогностическим фактором, связанным с инвазией и рецидивом опухоли [25]. Подавление экспрессии iNOS приводит к значительному снижению темпов роста клеток глиомы линии C6 [26].
Аргининдеиминаза (АДИ) – микробный фермент, который осуществляет гидролиз аргинина с образованием цитруллина и аммиака [27, 28]. Ферменты, полученные из Lactococcus lactis ssp. lactis, Pseudomonas plecoglossicida, Thermophilic Aspergillus fumigatus KJ434941, Mycoplasma arginini прошли и проходят множество клинических испытаний в разных странах в качестве препаратов для сочетанной терапии онкологических заболеваний [29–32]. Было показано, что конъюгированная с полиэтиленгликолем аргининдеиминаза (ADI-PEG20) через истощение аргинина индуцирует аутофагию и последующую гибель раковых клеток [33]. ADI-PEG20 хорошо зарекомендовала себя в клинических испытаниях II стадии у пациентов с гепатоцеллюлярной карциномой [34]. Кроме того, ADI-PEG20 показала высокую эффективность у пациентов с аргининосукцинатсинтетаза-1(ASS1)-негативной меланомой [35]. Наилучшие эффекты наблюдались при использовании ADI-PEG20 в сочетании с ингибитором аутофагии – хлорохином [36] или цитостатиками [37].
В данном исследовании изучали противоопухолевую активность стрептококковой АДИ в отношении клеток лимфобластной лейкемии линии Jurkat. Путем сравнения эффектов исходного штамма, экспрессирующего АДИ, и его изогенного мутанта с инактивированным геном АДИ – arcA было установлено, что стрептококковый фермент подавлял пролиферативную активность и усиливал аутофагию клеток без снижения их жизнеспособности.
МЕТОДЫ ИССЛЕДОВАНИЯ
Получение супернатантов разрушенных стрептококков
Исследование проводили с использованием Streptococcus pyogenes штамма М49-16 и его изогенного мутанта М49-16delArcA с инактивированным геном аргининдеиминазы arcA. Штаммы, а также супернатанты разрушенных стрептококков были получены и стандартизированы, как описано в [38], и любезно предоставлены профессором А.Н. Суворовым (Отдел молекулярной микробиологии ФБГНУ “ИЭМ”). Супернатанты разрушенных стрептококков (СРС) исходного и мутантного штаммов, содержащие внутриклеточные биологически активные вещества стрептококков, получали согласно описанной ранее методике [39].
Культивирование клеток линии Jurkat
Клетки линии Jurkat были получены из Российской коллекции клеточных культур позвоночных (Институт цитологии РАН). Линия Jurkat была выделена из крови больного Т-лимфобластной лейкемией. Клетки культивировали в среде RPMI-1640 (ПанЭко, Россия) с добавлением 10% инактивированной эмбриональной телячьей сыворотки (Invotrigen, США), 2 мМ глутамина (Биолот, Россия), 50 мкг/мл гентамицина (Биолот, Россия) (полная культуральная среда) во влажной атмосфере с 5%-ным содержанием СО2 при температуре 37°С. Пересев культуры осуществляли 1 раз в 3–4 дня. Кратность пересева составляла 1 : 5.
Влияние аргининдеиминазы на пролиферацию клеток Jurkat
Влияние стрептококковой АДИ на пролиферацию клеток Jurkat изучали в МТТ-тесте. Для этого 100 мкл клеточной суспензии в концентрации 1 млн/мл вносили в 96-луночный плоскодонный планшет (Sarstedt, Австрия). Здесь и далее действие АДИ на исследуемые параметры изучали путем сравнения СРС исходного и мутантного штаммов. СРС вносили в разведении 1/200 и 1/400 (v/v). Для подтверждения того, что действие фермента связано с деплецией аргинина, в среду для культивирования клеток добавляли L-аргинин (Sigma, США) в супрафизиологической концентрации 2 мМ. Клетки инкубировали 72 ч и на последние 4 ч в лунки вносили МТТ (3-(4,5-диметилтиазол-2-ил)-2,5-дифенил-тетразолиум бромид) (Sigma, США) в концентрации 0.5 мг/мл. Для лизиса клеток и растворения кристаллов формазана в лунки вносили 100 мкл лизирующего буфера, содержащего 10%-ный додецилсульфат натрия (Serva, Германия) в 0.01 N HCl и инкубировали 18 ч при 37°С. Оптическую плотность измеряли на спектрофотометре (Bio-Rad, Япония) при длине волны 570 нм. Оптическую плотность контрольных лунок принимали за 100% пролиферативной активности.
Для изучения распределения по фазам клеточного цикла клетки засевали в 12-луночный планшет (Sarstedt, Австрия) по 1 млн клеток в 1 мл полной культуральной среды и инкубировали с исследуемыми субстанциями 72 ч при 37°С во влажной атмосфере с 5%-ным содержанием СО2. Клетки пермеабилизировали 80%-ным ледяным метанолом в течение 5 мин. Для оценки доли клеток в фазах синтеза (S/G2) проводили окрашивание клеточной суспензии меченными FITC антителами против циклина А2 (Becman Coulter, США, кат. № PNA22327) в соответствии с рекомендациями производителя. Для оценки количества ДНК в клетках дополнительно проводили окрашивание клеточной суспензии ДНК-связывающим красителем DAPI (Invitrogen, США). Краситель вносили в концентрации 300 нM и инкубировали 10 мин. Анализ образцов проводили с использованием проточного цитофлуориметра Navios™ (Beckman Coulter, США). Распределение клеток по фазам клеточного цикла оценивали с помощью алгоритма Michael H. Fox, встроенного в программное обеспечение Kaluza Analysis 2.1 (США). Результаты выражали в процентах.
Влияние аргининдеиминазы на процессы аутофагии
Для оценки влияния СРС на процессы аутофагии в лунки 24-луночного планшета (Sarstedt, Австрия) вносили 500 мкл клеточной суспензии в концентрации 1 млн/мл. После этого добавляли СРС в разведении 1/400 (v/v) и L-аргинин (Sigma, США) в концентрации 2 мМ. По окончании 24-часовой инкубации пробы переносили в пробирки для цитометрии. Клетки осаждали путем центрифугирования при 300 g в течение 5 мин при комнатной температуре. Пробы окрашивали красителем Lysotracker Green DND-26 (Invitrogen, США) в концентрации 50 нМ. После 15-минутной инкубации проводили две отмывки клеток путем центрифугирования в фосфатно-солевом буфере (pH 7.0) при 300 g в течение 5 мин. Для оценки доли клеток в состоянии некроза и исключения их из анализа клеточную суспензию окрашивали ДНК-связывающим красителем DAPI в концентрации 300 нм. Пробы анализировали на проточном цитометре Navious (Beckman Coulter, США). Результаты выражали в средней интенсивности флуоресценции (mean fluorescence intensity, MFI).
Статистическая обработка
Статистическую обработку данных проводили с помощью программ Microsoft Office Excel 2010, Statistica 12.0, Kaluza Analysis 2.1 и GraphPad Prism 8. Для анализа нормальности распределения выборки использовали критерий Шапиро–Уилка. Далее выборку анализировали на наличие статистических значимых различий с помощью однофакторного дисперсионного анализа (ANOVA) в случае нормального распределения, или критерия Крускала–Уоллиса при ненормальном распределении. Затем проводили поиск попарных достоверных различий, при нормальном распределении использовали апостериорный тест Тьюки, при ненормальном распределении – критерий Данна. Данные в случае нормального распределения, представлены как среднее ± ошибка среднего (M ± SEM), при ненормальном распределении – как медиана [25 квартиль; 75 квартиль] (Med [Q25; Q75]). Во всех экспериментах различия считали значимыми при р < 0.05.
РЕЗУЛЬТАТЫ ИССЛЕДОВАНИЯ
Сравнение влияния супернатантов разрушенных стрептококков исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на пролиферативную активность клеток Jurkat
В присутствии СРС исходного штамма в разведениях 1/400 и 1/200 пролиферативная активность клеток статистически значимо снижалась по сравнению с контролем (рис. 1). СРС мутантного штамма в разведении 1/400 также статистически значимо, но слабее, чем исходный штамм подавлял пролиферацию. Введение добавки аргинина приводило к статистически значимому снижению исследуемого показателя в стандартных условиях культивирования и в присутствии СРС мутантного штамма. При этом добавка аргинина достоверно восстанавливала уровень пролиферации, подавленный под влиянием СРС исходного штамма. Для подтверждения этих результатов в дальнейших исследованиях изучали действие АДИ на распределение клеток по фазам клеточного цикла.
Рис. 1.
Сравнение влияния супернатантов разрушенных исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на пролиферативную активность клеток Jurkat. Пролиферативную активность изучали в МТТ-тесте. Данные представлены как Med [Q25; Q75], n = 18. Отличия статистически значимы по сравнению с контролем при ***p < 0.001; пролиферация клеток в присутствии СРС S. pyogenes М49-16delArcA достоверно выше, чем пролиферация клеток в присутствии СРС S. pyogenes М49-16, при ###р < 0.001; отличия статистически значимы по сравнению с этим параметром без добавки аргинина, при †† p < 0.01, † p < 0.05.
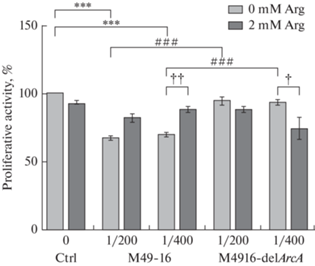
Анализ распределения клеток по фазам клеточного цикла показал, что в стандартных условиях большая часть (60.2 ± 3.2%) клеток находилась в фазе покоя (G0/G1), доля клеток в фазе синтеза (S) составляла 27.3 ± 2.7%, а доля клеток в фазе G2 и делящихся клеток (M) – 12.4 ± 3.4% (рис. 2). Доли клеток в фазах клеточного цикла в присутствии СРС мутантного штамма не отличались от этих показателей в контроле. В присутствии СРС исходного штамма происходило статистически значимое изменение доли клеток в фазах G0/G1 и S: доля клеток в фазах покоя увеличивалась на 10%, напротив, доля клеток в фазе синтеза уменьшалась на 7%. Добавление аргинина в культуру клеток, которые инкубировали в присутствии СРС исходного штамма, восстанавливало соотношение доли клеток в фазах клеточного цикла до значений близких к контролю.
Рис. 2.
Сравнение влияния супернатантов разрушенных исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на распределение клеток Jurkat по фазам клеточного цикла. Клеточную суспензию окрашивали ДНК-связывающим красителем DAPI. Здесь и далее образцы анализировали с использованием проточной цитометрии. Процент клеток в различных фазах клеточного цикла подсчитывали с использованием программного обеспечения для анализа Kaluza 2.2, основанного на алгоритме Майкла Х. Фокса. Данные представлены как M ± SEM, n = 18. Отличия статистически значимы: *p < 0.05 по сравнению с контролем; † p < 0.05 – по сравнению с этим параметром без добавки аргинина.
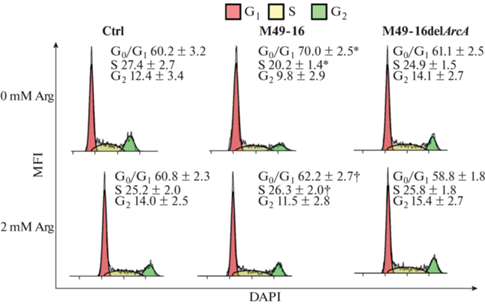
Далее проводили анализ экспрессии в клетках циклина А2, уровень которого строго синхронизирован с ходом клеточного цикла. Комплекс циклин – cyclin-dependent kinase 2 (A2-CDK2) – инициирует репликацию ДНК и необходим для прогрессирования S-фазы. Для прохождения через фазу G2 в фазу M циклин A2 образует комплексы с CDK1, после чего деградирует по убиквитин-зависимому пути протеолиза на ранних стадиях митоза [40]. Исследования подтвердили ранее полученные результаты. В частности, в контроле доля клеток в фазах синтеза S/G2 составляла приблизительно 40%. В присутствии СРС мутантного штамма значение этого параметра не отличалось от контроля. Культивирование клеток в присутствии СРС исходного штамма приводило к двукратному снижению доли клеток в фазах синтеза до 20%. Введение добавки аргинина полностью отменяло ингибирующее действие СРС исходного штамма, но не оказывало никакого действия на клетки, которые культивировали в стандартных условиях и в присутствии мутантного штамма.
Таким образом, с помощью МТТ-теста и анализа распределения клеток по фазам клеточного цикла и доли циклин А2-позитивных клеток было установлено, что стрептококковая АДИ подавляет пролиферацию клеток Jurkat за счет истощения аргинина в культуре.
Сравнение влияния супернатантов разрушенных стрептококков исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на интенсивность процессов аутофагии клеток Jurkat
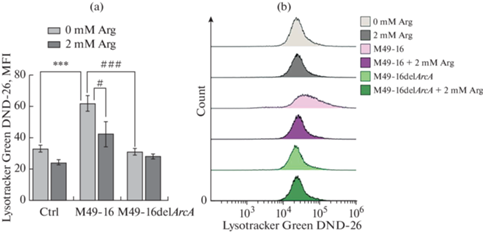
Дефицит нутриентов приводит к активации в клетках процессов аутофагии, которые призваны компенсировать недостающие аминокислоты и другие метаболиты [41]. Поэтому в дальнейших исследованиях проводили изучение влияния аргинидеиминазы на процессы аутофагии. Для этого использовали краситель LysoTracker Green DND-26, который накапливается в органеллах с низким уровнем рН, а интенсивность флуоресценции клеток отражает уровень процессов аутофагии [42]. Было установлено, что в присутствии СРС мутантного штамма интенсивность аутофагии не отличалась от таковой в контроле (рис. 4). При культивировании клеток в присутствии СРС исходного штамма происходило статистически значимое двукратное усиление интенсивности аутофагии по сравнению с этим показателем в стандартных условиях культивирования. При этом введение добавки аргинина снижало уровень аутофагии до значений этого параметра в контроле.
Рис. 3.
Сравнение влияния супернатантов разрушенных исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на долю циклин А2-позитивных клеток Jurkat. Репрезентативные проточно-цитометрические гистограммы, отражающие прогрессию клеточного цикла. (a) – культуральная среда (контроль); (b) – СРС M49-16; (c) – СРС M49-16delArcA; (d) – 2 мМ аргинина; (e) – СРС M49-16 и 2 мМ аргинина; (f) – СРС M49-16delArcA и 2 мМ аргинина; (g) – контроль изотипических антител. Проводили окрашивание клеточной суспензии меченными FITC антителами против циклина А2. Пробы анализировали на проточном цитометре Navious (Beckman Coulter) и программного обеспечения Kaluza Analysis 2.1. Данные представлены как M ± SEM, n = 5–6. Отличия статистически значимы при p < 0.001: *** – по сравнению с контролем, ### p < 0.001 – по сравнению с СРС исходного штамма S. pyogenes М49-16 (S. pyogenes M49-16).
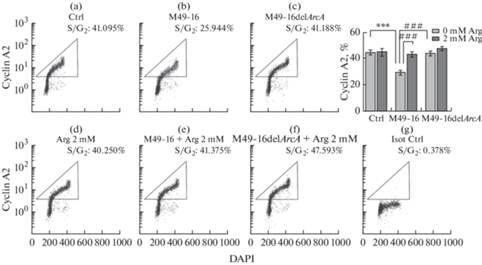
Рис. 4.
Сравнение влияния супернатантов разрушенных исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на процессы аутофагии клеток Jurkat. (a) – гистограмма, основанная на данных проточной цитометрии, показывающая количественные различия уровня аутофагии клеток Jurkat. (b) – репрезентативные гистограммы проточной цитометрии, отражающие уровень аутофагии клеток Jurkat. Пробы окрашивали Lysotracker DND-26 Green и анализировали методом проточной цитометрии. Данные представлены как M ± SEM, n = 4–9. Отличия статистически значимы: * p < 0.05 по сравнению с контролем, † – по сравнению с этим параметром без добавки аргинина.
Сравнение влияния супернатантов разрушенных стрептококков исходного (S. pyogenes M49-16) и мутантного (S. pyogenes M49-16delArcA) штаммов на жизнеспособность клеток Jurkat
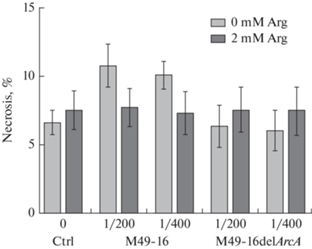
В дальнейших исследованиях изучали, не связано ли ингибирующее пролиферацию клеток действие АДИ, а также усиление процесса аутофагии, со снижением их жизнеспособности. Для этого проводили анализ доли клеток в состоянии некроза (рис. 5). Было установлено, что в присутствии СРС исходного штамма происходило слабое повышение доли клеток в состоянии некроза, однако эти изменения не были достоверными. В других условиях культивирования достоверного снижения жизнеспособности клеток тоже не было зарегистрировано.
ОБСУЖДЕНИЕ РЕЗУЛЬТАТОВ
Сопоставление эффектов СРС исходного и мутантного штаммов показало, что стрептококковая АДИ подавляет пролиферацию клеток линии Jurkat (рис. 1–3), что согласуется с данными, полученными ранее, с использованием АДИ других микробов. Так, в 2000 г. Gong и соавт. установили, что АДИ, выделенная из микоплазмы, ингибировала прохождение Jurkat по фазам клеточного цикла. Причем, было показано, что для блокировки прохождения клеточного цикла клеток Jurkat требовалась меньшая концентрация фермента, по сравнению с той, которая была необходима для первичных лимфоцитов. А высокие концентрации фермента (200 нг/мл) вызывали апоптоз клеток [43].
Позже было установлено, что культивирование Jurkat в течение 24 ч в среде, не содержащей аргинин, приводило к снижению экспрессии зета-цепи (CD3zeta) T-клеточного рецептора. Введение добавки аргинина восстанавливало уровень экспрессии этой молекулы до контрольных значений. Также при культивировании в дефицитной по аргинину среде наблюдалось значительное снижение пролиферации клеток. Таким образом, было установлено, что депривация аргинина может подавлять пролиферацию Jurkat за счет регуляции сигналов от Т-клеточного рецептора [44]. Антипролиферативное действие на клетки линии Jurkat было также описано для ADI-PEG20, выделенной из Vibrio alginolyticus [45].
Сопоставление полученных в нашей работе результатов с данными других исследователей показывают, что ингибирующее пролиферацию клеток Jurkat действие АДИ СРС S. pyogenes M49-16 было выражено слабее, если сравнивать с антипролиферативными эффектами фермента, выявленными в работах других авторов. Эти может быть прежде всего связано с тем, что ферменты, выделенные из разных микроорганизмов, отличаются по своим биохимическим характеристикам (оптимум pH, удельная активность, процессивность, сродство к субстрату). Также нужно учитывать некоторые методические отличия, как например, использование рекомбинатного белка, его коньгата с полиэтиленгликолем или фермента в составе супернатанта разрушенных стрептококков.
Также в литературе встречается ряд исследований, в которых изучали эффекты аргиназы или дефицитной по аргинину среды. Bernard и соавт. показали, что аргиназа эритроцитов значительно подавляла пролиферацию клеток Jurkat за счет снижения содержание аргинина в культуральной среде. Анализ с использованием селективного ингибитора Nor-NOHA показал, что подавление активности этого фермента восстанавливает пролиферацию клеток, обработанных эритроцитами [13].
В другой работе [46], изучали молекулярные механизмы нарушения функциональной активности лимфоцитов при их культивировании в дефицитной по аргинину среде. Было установлено, что в отсутствие аргинина происходила остановка в фазах G0/G1 клеточного цикла активированных митогеном Jurkat. Восполнение дефицита аминокислоты восстанавливало нормальный профиль клеточного цикла и пролиферацию клеток. Кроме того, депривация аргинина вызывала подавление экспрессии ряда мембранных антигенов, включая CD247, и развитие ER-стресса. С помощью генетического и биохимического подходов было подтверждено, что истощение аргинина также индуцировало развитие аутофагии клеток Jurkat. А ингибирование аутофагии при голодании клеток приводило к развитию апоптоза [46]. Результаты наших исследований согласуются с этими данными и показывают, что стрептококковая АДИ за счет создания дефицита аргинина запускает в клетках Jurkat процесс аутофагии (рис. 4), что, вероятно, спасает их от гибели, как подтверждают данные по оценке жизнеспособности (рис. 5).
В исследовании Di Marzio и соавт. изучали действие супернатантов разрушенных Streptococcus thermophilus и Lactobacillus brevis, авторами было установлено, что после 18 ч инкубации в присутствии бактериальных факторов уровень апоптоза в культуре клеток линии Jurkat возрастал до 86% по сравнению с 3% в контроле. Авторы предположили, что апоптоз был вызван высоким уровнем активности АДИ, которая, истощая субстрат для аргиназы, может подавлять синтез полиаминов, необходимых для пролиферации клеток [47]. Однако наши эксперименты с использованием разных методов оценки жизнеспособности клеток показали, что стрептококковая АДИ не вызывала значительного усиления гибели клеток в культуре (рис. 5).
Результаты данного исследования показывают, что стрептококковая АДИ обладает потенциальным противоопухолевым действием. Под влиянием фермента происходит подавление пролиферации клеток, что, вероятно, вызвано их голоданием. При этом не наблюдается усиления гибели клеток, но происходит компенсаторное усиление процессов аутофагии. Полученные данные подтверждает возможность использования аргинин-гидролизующей активности стрептококкового фермента для сочетанной терапии онкологических заболеваний.
Список литературы
Morris SM Jr (2016) Arginine Metabolism Revisited. J Nutrition 146: 2579S–2586S. https://doi.org/10.3945/jn.115.226621
Kirk SJ, Barbul A (1990) Role of arginine in trauma, sepsis, and immunity. JPEN J Parenter Enteral Nutr 14: 226S–229S. https://doi.org/10.1177/014860719001400514
Starikova EA, Rubinstein AA, Mammedova JT, Isakov DV, Kudryavtsev IV (2023) Regulated Arginine Metabolism in Immunopathogenesis of a Wide Range of Diseases: Is There a Way to Pass between Scylla and Charybdis? Current Issues Mol Biol 45: 3525–3551. https://doi.org/10.3390/cimb45040231
Morris CR (2014) Alterations of the arginine metabolome in sickle cell disease: a growing rationale for arginine therapy. Hematol Oncol Clin North Am 28: 301–321. https://doi.org/10.1016/j.hoc.2013.11.008
Morris CR, Kim H-Y, Klings ES, Wood J, Porter JB, Trachtenberg F, Sweeters N, Olivieri NF, Kwiatkowski JL, Virzi L, Hassell K, Taher A, Neufeld EJ, Thompson AA, Larkin S, Suh JH, Vichinsky EP, Kuypers FA, Thalassemia Clinical Research Network (2015) Dysregulated arginine metabolism and cardiopulmonary dysfunction in patients with thalassaemia. Br J Haematol 169: 887–898. https://doi.org/10.1111/bjh.13452
Morris CR (2008) Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program 177–185. https://doi.org/10.1182/asheducation-2008.1.177
Morris CR, Kuypers FA, Lavrisha L, Ansari M, Sweeters N, Stewart M, Gildengorin G, Neumayr L, Vichinsky EP (2013) A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 98: 1375–1382. https://doi.org/10.3324/haematol.2013.086637
Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM (2004) Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med 170: 148–153. https://doi.org/10.1164/rccm.200309-1304OC
Morris CR (2013) Arginine and asthma. Nestle Nutr Inst Workshop Ser 77: 1–15. https://doi.org/10.1159/000351365
Hsu C-N, Tain Y-L (2019) Impact of Arginine Nutrition and Metabolism during Pregnancy on Offspring Outcomes. Nutrients 11: 1452. https://doi.org/10.3390/nu11071452
Clark A, Imran J, Madni T, Wolf SE (2017) Nutrition and metabolism in burn patients. Burns & Trauma 5: 11. https://doi.org/10.1186/s41038-017-0076-x
Pribis JP, Zhu X, Vodovotz Y, Ochoa JB (2012) Systemic Arginine Depletion After a Murine Model of Surgery or Trauma. JPEN J Parenter Enteral Nutr 36: 53–59. https://doi.org/10.1177/0148607111414579
Bernard AC, Mistry SK, Morris SM, O’Brien WE, Tsuei BJ, Maley ME, Shirley LA, Kearney PA, Boulanger BR, Ochoa JB (2001) Alterations in arginine metabolic enzymes in trauma. Shock 15: 215–219. https://doi.org/10.1097/00024382-200115030-00009
Martí I Líndez A-A, Reith W (2021) Arginine-dependent immune responses. Cell Mol Life Sci 78: 5303–5324. https://doi.org/10.1007/s00018-021-03828-4
Albaugh VL, Pinzon-Guzman C, Barbul A (2017) Arginine-Dual roles as an onconutrient and immunonutrient. J Surg Oncol 115: 273–280. https://doi.org/10.1002/jso.24490
Zhao C, Guo H, Hou Y, Lei T, Wei D, Zhao Y (2023) Multiple Roles of the Stress Sensor GCN2 in Immune Cells. Int J Mol Sci 24: 4285. https://doi.org/10.3390/ijms24054285
Patil MD, Bhaumik J, Babykutty S, Banerjee UC, Fukumura D (2016) Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene 35: 4957–4972. https://doi.org/10.1038/onc.2016.37
Chen C-L, Hsu S-C, Ann DK, Yen Y, Kung H-J (2021) Arginine Signaling and Cancer Metabolism. Cancers (Basel) 13: 3541. https://doi.org/10.3390/cancers13143541
Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, Kundu M, Kim D-H (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20: 1992–2003. https://doi.org/10.1091/mbc.e08-12-1249
Szlosarek PW (2014) Arginine deprivation and autophagic cell death in cancer. Proc Natl Acad Sci U S A 111: 14015–14016. https://doi.org/10.1073/pnas.1416560111
Ishimwe N, Zhang W, Qian J, Zhang Y, Wen L (2020) Autophagy regulation as a promising approach for improving cancer immunotherapy. Cancer Lett 475: 34–42. https://doi.org/10.1016/j.canlet.2020.01.034
Hackett CS, Quigley DA, Wong RA, Chen J, Cheng C, Song YK, Wei JS, Pawlikowska L, Bao Y, Goldenberg DD, Nguyen K, Gustafson WC, Rallapalli SK, Cho Y-J, Cook JM, Kozlov S, Mao J-H, Van Dyke T, Kwok P-Y, Khan J, Balmain A, Fan Q, Weiss WA (2014) Expression quantitative trait loci and receptor pharmacology implicate Arg1 and the GABA-A receptor as therapeutic targets in neuroblastoma. Cell Rep 9: 1034–1046. https://doi.org/10.1016/j.celrep.2014.09.046
Sousa MSA, Latini FRM, Monteiro HP, Cerutti JM (2010) Arginase 2 and nitric oxide synthase: Pathways associated with the pathogenesis of thyroid tumors. Free Radic Biol Med 49: 997–1007. https://doi.org/10.1016/j.freeradbiomed.2010.06.006
Yu Y, Ladeiras D, Xiong Y, Boligan KF, Liang X, von Gunten S, Hunger RE, Ming X-F, Yang Z (2020) Arginase-II promotes melanoma migration and adhesion through enhancing hydrogen peroxide production and STAT3 signaling. J Cell Physiol 235: 9997–10011. https://doi.org/10.1002/jcp.29814
Belgorosky D, Girouard J, Langle YV, Hamelin-Morrissete J, Marino L, Agüero EI, Malagrino H, Reyes-Moreno C, Eiján AM (2020) Relevance of iNOS expression in tumor growth and maintenance of cancer stem cells in a bladder cancer model. J Mol Med (Berl) 98: 1615–1627. https://doi.org/10.1007/s00109-020-01973-0
Girotti AW, Fahey JM, Korytowski W (2020) Nitric oxide-elicited resistance to anti-glioblastoma photodynamic therapy. Cancer Drug Resist 3: 401–414. https://doi.org/10.20517/cdr.2020.25
Gallego P, Planell R, Benach J, Querol E, Perez-Pons JA, Reverter D (2012) Structural characterization of the enzymes composing the arginine deiminase pathway in Mycoplasma penetrans. PLoS One 7: e47886. https://doi.org/10.1371/journal.pone.0047886
Hirose Y, Yamaguchi M, Sumitomo T, Nakata M, Hanada T, Okuzaki D, Motooka D, Mori Y, Kawasaki H, Coady A, Uchiyama S, Hiraoka M, Zurich RH, Amagai M, Nizet V, Kawabata S (2021) Streptococcus pyogenes upregulates arginine catabolism to exert its pathogenesis on the skin surface. Cell Rep 34: 108924. https://doi.org/10.1016/j.celrep.2021.108924
Zhang L, Liu M, Jamil S, Han R, Xu G, Ni Y (2015) PEGylation and pharmacological characterization of a potential anti-tumor drug, an engineered arginine deiminase originated from Pseudomonas plecoglossicida. Cancer Lett 357: 346–354. https://doi.org/10.1016/j.canlet.2014.11.042
Abou-Alfa GK, Qin S, Ryoo B-Y, Lu S-N, Yen C-J, Feng Y-H, Lim HY, Izzo F, Colombo M, Sarker D, Bolondi L, Vaccaro G, Harris WP, Chen Z, Hubner RA, Meyer T, Sun W, Harding JJ, Hollywood EM, Ma J, Wan PJ, Ly M, Bomalaski J, Johnston A, Lin C-C, Chao Y, Chen L-T (2018) Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 29: 1402–1408. https://doi.org/10.1093/annonc/mdy101
Wei J, Bera TK, Liu XF, Zhou Q, Onda M, Ho M, Tai C-H, Pastan I (2018) Recombinant immunotoxins with albumin-binding domains have long half-lives and high antitumor activity. Proc Natl Acad Sci U S A 115: E3501–E3508. https://doi.org/10.1073/pnas.1721780115
Li R, Yang H, Jia D, Nie Q, Cai H, Fan Q, Wan L, Li L, Lu X (2016) Fusion to an albumin-binding domain with a high affinity for albumin extends the circulatory half-life and enhances the in vivo antitumor effects of human TRAIL. J Control Release 228: 96–106. https://doi.org/10.1016/j.jconrel.2016.03.004
Changou CA, Chen Y-R, Xing L, Yen Y, Chuang FYS, Cheng RH, Bold RJ, Ann DK, Kung H-J (2014) Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc Natl Acad Sci U S A 111: 14147–14152. https://doi.org/10.1073/pnas.1404171111
Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, Cremona F, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA, Ng C, Curley SA (2004) Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 22: 1815–1822. https://doi.org/10.1200/JCO.2004.11.120
Feun LG, Marini A, Walker G, Elgart G, Moffat F, Rodgers SE, Wu CJ, You M, Wangpaichitr M, Kuo MT, Sisson W, Jungbluth AA, Bomalaski J, Savaraj N (2012) Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer 106: 1481–1485. https://doi.org/10.1038/bjc.2012.106
Syed N, Langer J, Janczar K, Singh P, Lo Nigro C, Lattanzio L, Coley HM, Hatzimichael E, Bomalaski J, Szlosarek P, Awad M, O’Neil K, Roncaroli F, Crook T (2013) Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis 4: e458. https://doi.org/10.1038/cddis.2012.197
Savaraj N, Wu C, Li Y-Y, Wangpaichitr M, You M, Bomalaski J, He W, Kuo MT, Feun LG (2015) Targeting argininosuccinate synthetase negative melanomas using combination of arginine degrading enzyme and cisplatin. Oncotarget 6: 6295–6309.
Starikova EA, Sokolov AV, Vlasenko AY, Burova LA, Freidlin IS, Vasilyev VB (2016) Biochemical and biological activity of arginine deiminase from Streptococcus pyogenes M22. Biochem Cell Biol 94: 129–137. https://doi.org/10.1139/bcb-2015-0069
Starikova EA, Golovin AS, Vasilyev KA, Karaseva AB, Serebriakova MK, Sokolov AV, Kudryavtsev IV, Burova LA, Voynova IV, Suvorov AN, Vasilyev VB, Freidlin IS (2019) Role of arginine deiminase in thymic atrophy during experimental Streptococcus pyogenes infection. Scand J Immunol 89: e12734. https://doi.org/10.1111/sji.12734
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, Yu M, Lin J, Cui Q (2020) The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int J Mol Sci 21: 1960. https://doi.org/10.3390/ijms21061960
He L, Zhang J, Zhao J, Ma N, Kim SW, Qiao S, Ma X (2018) Autophagy: The Last Defense against Cellular Nutritional Stress. Adv Nutr 9: 493–504. https://doi.org/10.1093/advances/nmy011
Chikte S, Panchal N, Warnes G (2014) Use of LysoTracker dyes: A flow cytometric study of autophagy. Cytometry Part A 85: 169–178. https://doi.org/10.1002/cyto.a.22312
Gong H, Zölzer F, von Recklinghausen G, Havers W, Schweigerer L (2000) Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia 14: 826–829. https://doi.org/10.1038/sj.leu.2401763
Taheri F, Ochoa JB, Faghiri Z, Culotta K, Park HJ, Lan MS, Zea AH, Ochoa AC (2001) L-Arginine regulates the expression of the T-cell receptor zeta chain (CD3zeta) in Jurkat cells. Clin Cancer Res 7: 958s–965s.
Unissa R, Sudhakar M, Reddy ASK (2016) Evaluation of in vitro Anti-proliferative Activity of L‑arginine deiminase from Novel Marine Bacterial Isolate. Microbiol Res J Int 1–10. https://doi.org/10.9734/BMRJ/2016/23592
García-Navas R, Munder M, Mollinedo F (2012) Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy 8: 1557–1576. https://doi.org/10.4161/auto.21315
Di Marzio L, Russo FP, D’Alò S, Biordi L, Ulisse S, Amicosante G, De Simone C, Cifone MG (2001) Apoptotic effects of selected strains of lactic acid bacteria on a human T leukemia cell line are associated with bacterial arginine deiminase and/or sphingomyelinase activities. Nutr Cancer 40: 185–196. https://doi.org/10.1207/S15327914NC402_16
Дополнительные материалы отсутствуют.
Инструменты
Российский физиологический журнал им. И.М. Сеченова