Сенсорные системы, 2020, T. 34, № 4, стр. 283-298
Психофизические и нейрофизиологические характеристики оценки наклонных ориентаций у мужчин и женщин
Е. С. Михайлова 1, *, Н. Ю. Герасименко 1, А. Б. Кушнир 1
1 ФГБУН Институт высшей нервной деятельности и нейрофизиологии РАН
117485 Москва, ул. Бутлерова, 5А, Россия
* E-mail: esmikhailova@mail.ru
Поступила в редакцию 11.05.2020
После доработки 28.05.2020
Принята к публикации 16.07.2020
Аннотация
В работе исследовали половые различия дискриминации наклонных ориентаций. Задачу определения близости наклонных ориентаций к горизонтальному, вертикальному и наклонному (45°) референтам выполняли 34 испытуемых (16 мужчин и 18 женщин) с нормальным зрением. Регистрировали точность, время реакции и вызванные потенциалы каудальных областей коры. Показано, что женщины совершают больше ошибок по сравнению с мужчинами, но не обнаруживают различий во времени реакции. Выявлены половые различия раннего анализа наклонных ориентаций. Только в группе мужчин амплитуда ранней негативности N1 ВП затылочной коры зависела от наклона линий: минимальные значения амплитуды характерны для ответа на наклонные линии, близкие к кардинальным осям, максимальные – на линии, близкие к 45°. Предполагается, что в основе половых различий ранней чувствительности затылочной коры к наклонным ориентациям лежат особенности переработки информации в дорзальном и вентральном зрительных путях, определяемые пре- и постнатальным влиянием стероидных гормонов (Handa, McGivern, 2014).
ВВЕДЕНИЕ
Ориентация линейного отрезка внешнего контура изображения или контура его значимых фрагментов считается признаком первого порядка, на котором базируются операции раннего анализа формы зрительного объекта – сегментация, выделение и кодирование элементарных признаков. Детектирование ориентации является первой принципиальной операцией по анализу формы изображения и происходит на уровне первичных зрительных областей коры. Ее нейрофизиологические механизмы детально исследованы в экспериментах на животных, открыты специализированные нейроны-детекторы ориентаций, изучены их функциональные характеристики, особенности корковой топографии (Hubel, Wiesel, 1962). В 1966 г. Кемпбелл и Куликовски (Campbell, Kulikowski, 1966) открыли эффект промежуточных ориентаций, или “oblique” эффект, который проявлялся в лучшей контрастной чувствительности при ориентации стимулов вдоль кардинальных осей. В последующем преимущество кардинальных ориентаций было описано для широкого круга зрительных задач (Appelle, 1972).
В ранних исследованиях авторы стремились установить прямое соответствие между характеристиками корковых реакций на базовые и наклонные ориентации и поведенческими ответами. Так, в работе (Furmanski, Engel, 2000) описан классический “oblique” эффект в виде большего метаболического (BOLD) ответа в области V1 на базовые ориентации по сравнению с наклонными, который сочетался с лучшим выделением горизонталей и вертикалей по сравнению с наклонными ориентациями и более низкими порогами контрастной чувствительности для стимулов, ориентированных вдоль кардинальных осей. Проявления “oblique” эффекта выявлены также в областях коры, которые обрабатывают информацию о сценах (“scene-selective” Parahippocampal Place Area – PPA) (Nasr, Tootell, 2012). Причем эта преференция ответов на кардинальные ориентации наблюдалась как для натуральных сцен, так и для генерируемых компьютером стимулов, представляющих собой набор отрезков линий базовых и наклонных ориентаций. Таким образом, оценка ориентационных характеристик стимула не ограничивается анализом контура объекта, а селективная чувствительность к кардинальным осям свойственна не только ретинотопическим областям коры.
Предпочтение кардинальных ориентаций является устойчивой характеристикой зрительного восприятия, однако ряд авторов указывают на ее инверсию. В работе (Hansen, Essock, 2004) описано такое явление как “горизонтальный эффект”, проявляющееся в перцептивной недооценке превалирующих в естественной среде кардинальных ориентаций. Измененный “oblique” эффект наблюдали также для зашумленных стимулов (Yang et al., 2012) и при саккадических движениях глаз (Lee, Lee, 2008).
Возможные нейрофизиологические механизмы поведенческого “oblique” эффекта обсуждаются в исследованиях с применением метода вызванных потенциалов (Proverbio et al., 2002; Koelewijn et al., 2011; Takács et al., 2013; Михайлова и др., 2015). Так, например, Такас и соавт. (Takács et al., 2013) зарегистрировали “oblique” эффект в виде более высокой амплитуды волны рассогласования (MMN) при смене двух кардинальных ориентаций по сравнению со сменой двух наклонных, причем даже в случае, когда наклон линий был характеристикой нерелевантной выполняемой задаче. Это позволило авторам отнести “oblique” эффект к фундаментальным свойствам зрительного восприятия. Хотя поведенческий “oblique” эффект достаточно устойчив, его электрографические проявления могут иметь разный знак в зависимости от этапа переработки информации. Так, например, в исследовании (Koelewijn et al., 2011) авторы, используя метод регистрации магнитоэнцефалограммы, обладающей более высоким по сравнению с функциональной магниторезонансной томографией временным разрешением, показали, что на ранних этапах анализа (80 мс после начала стимула) ответ на наклонные ориентации превышал ответ на кардинальные, а позже (120 мс) регистрировали классический более мощный ответ на кардинальные ориентации. Зависимость знака “oblique” эффекта от этапа переработки информации показана в нашем исследовании (Mikhailova et al., 2018). На сенсорных этапах переработки в проекционных областях коры амплитуда ранних компонентов ВП выше на наклонные ориентационные решетки, а поздние компоненты в центральных и префронтальных областях, напротив, демонстрировали более мощный ответ на горизонтали и вертикали.
Ряд поведенческих феноменов указывает на различия в механизмах идентификации базовых и наклонных ориентаций. По данным исследования (Bloem et al., 2017), при уменьшении ресурсов внимания контрастная чувствительность ухудшается только для наклонных ориентаций. В работе (Yashar, Denison, 2017) сообщается о разной динамике перцептивного научения для ориентаций, близких к кардинальным и наклонным ориентирам. В работе (Dragoi et al., 2001) исследовали влияние процедуры адаптации на психофизиологические реакции человека и на ответы нейронов первичной зрительной коры мозга кошек. Была обнаружена более высокая устойчивость ответов на кардинальные ориентации по сравнению с наклонными. По мнению авторов работы (Dragoi et al., 2001), это свойство ориентационной настройки нейронов детекторов горизонталей и вертикалей является важным фактором, ограничивающим перекалибровку зрительной системы относительно кардинальных осей, и препятствует искажению зрительного восприятия, возникающему при некоторых внешних воздействиях.
В литературе широко представлены сведения о половых различиях зрительно-пространственной деятельности. Как правило, они касаются сложных, включающих когнитивное звено, форм поведения, таких, например, как навигация, конструирование, трехмерное вращение (Butler et al., 2006; Ocklenburg et al., 2011; Mikhailova et al., 2012; Gagnon et al., 2018; Harris et al., 2019; Nazareth et al., 2019). Половым различиям выполнения более простых зрительных задач уделено меньше внимания. По этой теме есть лишь единичные работы (Collaer, Nelson, 2002; Caparelli-Dáquer et al., 2009; Славуцкая и др., 2014). Исследование зрительного восприятия с применением батареи из 15 тестов (острота зрения, порог контрастной чувствительности, оценка направления движения, ориентационная чувствительность, зрительные иллюзии и другое) на большой когорте испытуемых показало отчетливое преимущество мужчин в их выполнении (Shaqiri et al., 2018).
Ранее мы описали отчетливые половые различия в величине раннего ответа зрительных областей коры в задаче идентификации базовых и двух наклонных ориентаций 45 и 135° (Михайлова и др., 2015). Однако эти две ориентации не исчерпывают всего многообразия наклонных линий в естественной среде, и многие реальные зрительные задачи связаны с оценкой более широкого диапазона наклонных линий. В некоторых ситуациях возникает необходимость не точной оценки ориентации, а определения ее расхождения с базовыми направляющими осями зрительного пространства.
Цель настоящей работы – по данным регистрации вызванной активности коры мозга провести анализ ранних этапов переработки информации о наклонных ориентациях и сравнить их характеристики в группах мужчин и женщин. Ранее на большой группе испытуемых мы исследовали половые различия определения наклонных ориентаций в бланковом тесте Бентона (Славуцкая и др., 2014). Специфика выполнения теста Бентона не позволяет воспроизвести его в эксперименте с регистрацией вызванных потенциалов. Поэтому для решения поставленной цели мы использовали задачу определения близости набора тестовых наклонных линий к трем референтам: горизонтали, вертикали и наклонной ориентации 45°. Сходная экспериментальная модель анализа ориентационной чувствительности использована в работах (Edden et al., 2009; Patten et al., 2017). Наше исследование проведено на испытуемых, ранее выполнявших тест Бентона и показавших результаты, относящиеся к центральным квартилям распределения оценок правильности выполнения теста (Славуцкая и др., 2014). Полученные в этом исследовании результаты указывают на возможность связанных с полом различий в выделении и кодировании информации о широком спектре наклонных ориентаций.
ОПИСАНИЕ МЕТОДИКИ
В исследовании участвовали 34 испытуемых c нормальным зрением (18 женщин и 16 мужчин, средний возраст – 22 и 24 года соответственно). У всех испытуемых, согласно их самоотчету, предпочитаемой рукой была правая. Во всех случаях было получено письменное согласие испытуемых на проведение исследования соответственно протоколу, утвержденному этической комиссией ИВНД и НФ РАН. Исследования проводили в первой половине дня c 9 до 14 ч.
Стимулы представляли собой прямоугольные контрастные решетки из черных линий на светло-сером фоне (1 цикл/град.). Соотношение толщины черных и светло-серых линий 1: 2. Использовали восемь решеток линий с наклоном по часовой стрелке на 9, 18, 27, 36, 54, 63, 72 и 81° от вертикали. Примеры стимулов приведены на рис. 1, а. Размер стимула на экране – 11 см, что при расстоянии 120 см от экрана до глаз испытуемого составляло 5.5 угл. град.
Рис. 1.
Условия эксперимента.
а – зрительные стимулы – ориентационные решетки с разным углом наклона тестовых линий. б – схема расположения регистрирующих каудальных электродов на скальпе. Квадрат – референтный электрод (Cz). Обведены зона затылочного кластера и электроды, для которых проведен анализ ВП.
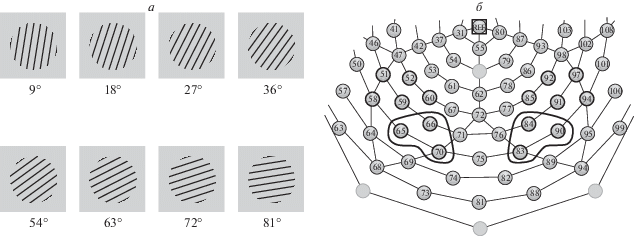
Во время исследования испытуемый сидел в удобном кресле в затемненной и звукозаглушенной комнате на расстоянии 120 см от экрана монитора Dell E1911c (диагональ 17 дюймов, разрешение в пикселях1280*1024, частота обновления экрана 60 Гц). Освещенность на уровне глаз испытуемого – 3 лк. Горизонтальные и вертикальные края монитора были закрыты экраном с закругленными краями и круглой апертурой. Стимулы предъявляли бинокулярно в центр экрана монитора при помощи программы E-Prime 2.0 (Psychology Software Tools, Inc., США). Длительность стимула 100 мс, межстимульный интервал в случайном порядке изменялся от 3 до 4 с. Каждый стимул предъявляли по 34 раза, последовательность стимулов – случайная.
Задача испытуемого представляла собой процедуру трехальтернативного выбора и заключалась в определении близости предъявляемых на экране наклонных линий к горизонтали, вертикали или к 45°. Ответом была моторная реакция – нажатие той клавиши выносной клавиатуры Serial Response Box, которая по инструкции соответствовала одному их трех референтов. Близкими к вертикали были решетки с углом наклона 9 и 18°, близкими к горизонтали – 72 и 81°, близкими к 45° – 27, 36, 54 и 63°. Регистрировали время реакции и правильность ответа испытуемого. Для каждого испытуемого рассчитывали точность опознания (в %) и среднее время реакции (ВР) правильных ответов для каждой из восьми ориентаций. До эксперимента испытуемым показывали стимулы, но предварительного обучения не проводили. Перед основным экспериментом в короткой обучающей серии испытуемых тренировали нажимать на клавиши выносной клавиатуры.
Регистрацию электроэнцефалограммы (ЭЭГ) осуществляли на 128-канальном энцефалографе фирмы Geodesics (Electrical Geodesics Inc., USA) при помощи шлема HydroCel Geodesic Sensor Net (Electrical Geodesics Inc., США). Частота квантования сигнала составляла 500 Гц. Сопротивление электродов не превышало 50 кОм, что соответствовало инструкции производителя (Electrical Geodesics Inc., USA). Полоса частот усилителя ограничивалась сверху 70 Гц, а снизу – 0.1 Гц. Использовали узкополосный заграждающий фильтр для частоты 50 Гц (notch фильтр). При записи ЭЭГ в качестве индифферентного электрода использовали электрод Cz, расположенный на вертексе. Для каждого испытуемого размер шлема Sensor Net подбирали в соответствии с размером его головы. После регистрации ЭЭГ проводили процедуру фоторегистрации положения электродов на голове испытуемого (блок Photogrammetry). Полученные индивидуальные данные о позиции электродов программно (Net Station 4.5.4) совмещали с данными регистрации ЭЭГ.
Данные ЭЭГ обрабатывались off-line в программе NetStation 4.5.4 (Electrical Geodesics Inc., США). Согласно рекомендациям производителя электроэнцефалографа, при обработке записи в качестве референтного электрода выбирали объединенный мастоидный электрод. Предварительно фильтрованные (0.5–45 Гц) записи ЭЭГ каждого испытуемого сегментировали на отрезки длительностью 1000 мс, содержащие интервалы 300 мс до предъявления стимула и 700 мс после. Далее программно исключали реализации с выраженными глазными движениями, а при дальнейшем визуальном анализе исключали фрагменты с другими артефактами (ЭМГ, двигательная активность). После этого для каждого испытуемого по 23–34 безартефактным отрезкам ЭЭГ проводили усреднение вызванных потенциалов (ВП) для правильных ответов на каждую ориентацию. Осуществляли коррекцию базовой линии по 300 мс отрезку ЭЭГ до предъявления стимула.
Амплитуды ранних компонентов (Р1 и N1) индивидуальных ВП измеряли в программе NetStation 4.5.4 от 16 каудальных электродов (в левом полушарии №№ 51, 52, 58, 59, 60, 65, 66, 70, в правом – №№ 83, 84, 85, 90, 91, 92, 96, 97). Во временных окнах этих компонентов (50–130 мс после начала стимула для P1, 100–190 мс для N1) замеряли адаптивный минимум или максимум компонентов, а именно средние значения амплитуды в 4-миллисекундном интервале, соответствующем 2 мс до и после пика компонента.
Статистический анализ амплитуд компонентов индивидуальных ВП, измеренных для каждого электрода, проводили с использованием дисперсионного анализа ANOVA RM (метод повторных измерений). Результаты анализа при необходимости были скорректированы поправкой Гринхауса-Гейссера. При post-hoc сопоставлениях использовали поправку Tukey на множественные сравнения, а для межгрупповых сопоставлений – тест Стьюдента.
РЕЗУЛЬТАТЫ
Анализ психофизических характеристик
Проведен дисперсионный анализ ANOVA RM времени реакции (ВР) и точности выполнения задачи отнесения тестовой ориентации к категориям “ближе к вертикали”, “ближе к горизонтали” и “ближе в наклонной 45°” с факторами “ориентация” и “пол”.
Для точности выявлено влияние фактора “ориентация” (F7,2240 = 23.10; p < 0.0005). Как видно на рис. 2, а, точность определения близости наклонных ориентаций к референтным осям зависит от степени их удаленности от осей. Наибольшая точность определения выявлена для 9 и 81° (97.1 ± 0.8% и 92.3 ± 1.4% соответственно), а более трудными для определения были ориентации 27 и 72° (63.9 ± 4.3% и 54.6± 4.6% соответственно), которые располагались близко к биссектрисе угла между наклонной и базовой осями. Анализ контрастов (Tukey test) подтвердил значимость представленных на рис. 2, а различий (табл. 1, ячейки под диагональю). Для ориентаций 27 и 72° точность выполнения задачи значимо ниже, чем для всех остальных вариантов наклона (p < 0.0001 для всех контрастов, кроме 72 и 18°, где p < 0.01). Наибольшая точность показана для ориентаций 9 и 81°, которая выше по сравнению с другими наклонами (p < 0.0001). Для точности показано значимое влияние фактора “пол” (F1, 32 = 6.74; p < 0.05). Среднее по всем ориентациям значение точности у мужчин соответствовало 82.3±5.1%, у женщин – 78.8±5.9%, различия значимы при T = 2.59; df = 32 (p = 0.014).
Рис. 2.
Психофизические характеристики оценки близости тестовых наклонных линий к горизонтали, вертикали и 45°.
а – точность оценки; б – ВР оценки. Темные столбики – данные в группе мужчин; светлые столбики – данные в группе женщин; в – точность оценки “легких” и “трудных” ориентаций; г – ВР оценки “легких” и “трудных” ориентаций. Темные столбики – данные для “легких” ориентаций (9, 36 и 54 и 81°). Светлые столбики – данные для “трудных” ориентаций (18, 27, 63 и 72°). По вертикали – точность оценки (%), по горизонтали – угол наклона ориентации в тестовом стимуле. На фрагменте в обозначены горизонтальный и вертикальный референты. Приведены средние по группе значения и ошибки среднего. Достоверность статистических различий: *** – р < 0.001, * – р < 0.05.
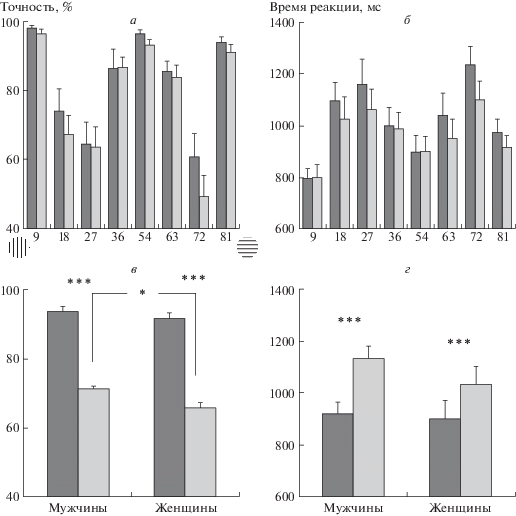
Таблица 1.
Результаты post-hoc анализа (Tukey test) ВР и точности.
| Град. | 9° | 18° | 27° | 36° | 54° | 63° | 72° | 81° |
|---|---|---|---|---|---|---|---|---|
| 9° | p < 0.0001 | p < 0.0001 | p < 0.0005 | – | p < 0.0001 | p < 0.0001 | p < 0.05 | |
| 18° | p < 0.0001 | – | – | p < 0.005 | – | – | – | |
| 27° | p < 0.0001 | – | – | p < 0.001 | – | – | p < 0.05 | |
| 36° | – | p < 0.01 | p < 0.0001 | – | – | p < 0.05 | – | |
| 54° | – | p < 0.0001 | p < 0.0001 | – | – | p < 0.0001 | – | |
| 63° | – | p < 0.05 | p < 0.0001 | – | – | p < 0.05 | – | |
| 72° | p < 0.0001 | p < 0.01 | – | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| 81° | – | p < 0.0001 | p < 0.0001 | – | – | – | p < 0.0001 |
Для ВР также выявлено значимое влияние фактора “ориентация” (F7,210 = 20.45; p < 0.0005). Как и для точности, ВР определения близости тестовых ориентаций к базовым и наклонной 45° осям зависит от степени их удаленности от референтов. Как видно на рис. 2, б, самое низкое значение ВР отмечено для близкой к вертикали ориентации 9° (798.7 ± 30.0 мс), а самое высокое – для ориентации 72° (1166.7 ± 49.8 мс), расположенной между горизонтальным и наклонным референтами. Как видно из табл. 1 (ячейки выше диагонали), ВР для ориентаций 9 и 72° отличались от ВР для большинства других вариантов наклона. Для ВР эффект фактора “пол” был статистически незначимым (p = 0.486).
На рис. 2, а, б видно, что гистограммы зависимости точности и ВР от угла наклона линии несимметричны. Точность для близкой к вертикали ориентации 18° значимо выше, чем для симметричной для нее ориентации 72°, расположенной рядом с горизонталью (p < 0.01, Tukey test). Сходным образом, для близкой к вертикали ориентации 9° ВР значимо меньше, чем для симметричной ей близкой к горизонтали ориентации 81° (p < 0.05, Tukey test). Разная зависимость от референтов “горизонталь” и “вертикаль” проявляется в характере ошибок. “Трудная” ориентация 27°, расположенная ближе к референту 45°, в трети случаев (36.1 ± 4.3%) оценивается ошибочно и идентифицируется как близкая к вертикали, а “трудная” ориентация 72°, близкая к горизонтали, почти в половине случаев (45.4% ± 4.5) ошибочно оценивается как расположенная ближе к 45°.
Для уточнения влияния близости тестовой ориентации к референтам мы разделили ориентации на две группы. В первую “близкую” группу включили ориентации 9, 36 и 54 и 81°. Во вторую “дальнюю” группу включили ориентации 18, 27, 63 и 72°. Значения точности и ВР были усреднены по группам ориентаций. Для полученных значений проведен дисперсионный анализ ANOVA RM с использованием факторов “близость” и “пол”.
Для точности выявлен эффект факторов “близость” (F1, 32 = 327.33; p < 0.0005) и “пол” (F1, 32 = = 6.74; p < 0.01). Тестовые ориентации, близкие к референтам, опознаются с большей точностью по сравнению с удаленными (p < 0.0005, Tukey test). При этом удаленные от референтов ориентации опознаются женщинами хуже, чем мужчинами (T = 2.42, df = 32, р < 0.05).
Для ВР получен эффект “близость” (F1, 32 = = 83.46; p < 0.0005) и взаимодействие “близость” × “пол” (F1, 32 = 4.55; p < 0.05). Удаленные ориентации опознаются медленнее, чем близкие (p < 0.0005, Tukey test). У мужчин это замедление более отчетливо, чем у женщин.
Анализ вызванных потенциалов
Анализ амплитуды компонентов Р1 и N1 проведен для ответов на наклонные решетки 9, 18, 36, 54, 63 и 81°. Данные для решеток 27 и 72° исключены из анализа из-за малого числа накоплений при правильных ответах.
Анализ амплитуды компонента Р1 не выявил значимых эффектов ни для одного из факторов (“ориентация”, “электрод”, “полушарие” и “пол”) .
Анализ амплитуды компонента N1. На первом этапе анализа был проведен дисперсионный анализ амплитуды компонента N1 с учетом факторов внутрииндивидуальной вариабельности “ориентация”, “электрод”, “полушарие” и фактора меж-индивидуальной вариабельности “пол”. Выявлен значимый эффект фактора “электрод” (F7,224 = = 12.33; p < 0.0005) и его зависимость от “полушария” в виде взаимодействия “электрод” × “полушарие” (F7,224 = 2.77; p < 0.05). Влияние “ориентации” на амплитуду N1 проявлялось в виде взаимодействий “ориентация” × “электрод” (F35,1120 = 18.80; p < 0.0005) и “ориентация” × × “полушарие” (F5,160 = 7.23; p < 0.0005), хотя основной эффект “ориентации” не достигал уровня значимости (F5,160 = 2.87; p = 0.06). Основной эффект фактора “пол” незначимым (F1,32 = 1.93; p = 0.18), но половые различия проявлялись в виде взаимодействий “ориентация” × “пол” (F5,160 = = 4.28; p < 0.001) и “ориентация” × “полушарие” × × “пол” (F5,160 = 5.91; p < 0.0005).
С учетом полученных данных об основных эффектах факторов и их взаимодействии был проведен дальнейший анализ амплитуды N1 на каждом из электродов с факторами “ориентация” и “пол”. Результаты анализа приведены в табл. 2. Обращает внимание межполушарная асимметрия эффектов. Для электродов правого полушария влияние “ориентации” зависело от “пола”, что проявлялось как взаимодействие “ориентация” × “пол” (0.0001 < p < 0.05). Для электродов левого полушария выявлено значимое влияние “ориентации” (0.001 < p < 0.05). Как видно из этой же таблицы, наиболее отчетливое влияние “наклона” и значимые взаимодействия “ориентация” × “пол” получены для амплитуды N1 ВП на электродах затылочного кластера, в правом полушарии это сенсоры №№ 83, 84 и 90, в левом полушарии – №№ 65, 66, 70. Их расположение приведено на рис. 1, б.
Таблица 2.
Результаты дисперсионного анализа ANOVA RM амплитуды N1 ВП, зарегистрированных на отдельных электродах. Приведены только значимые эффекты
| Полушарие | № электрода | Наклон | Наклон × Пол | ||||
|---|---|---|---|---|---|---|---|
| F | df | Sig. | F | df | Sig. | ||
| Левое | 52 | 2.88 | 5.160 | 0.03 | – | – | – |
| 59 | 3.42 | 5.160 | 0.01 | – | – | – | |
| 60 | 3.34 | 5.160 | 0.02 | – | – | – | |
| 65 | 2.68 | 5.160 | 0.02 | 2.26 | 5.160 | 0.05 | |
| 66 | 5.25 | 5.160 | 0.001 | – | – | – | |
| 70 (О1) | 4.49 | 5.160 | 0.002 | – | – | – | |
| Правое | 83 (О2) | 2.89 | 5.160 | 0.01 | 5.1 | 5.160 | 0.0001 |
| 84 | – | – | – | 2.87 | 5.160 | 0.02 | |
| 85 | – | – | – | 2.78 | 5.160 | 0.02 | |
| 90 | – | – | – | 4.03 | 5.160 | 0.002 | |
| 91 | – | – | – | 3.15 | 5.160 | 0.02 | |
| 96 | – | – | – | 2.59 | 5.160 | 0.03 | |
| 97 | – | – | – | 2.66 | 5.160 | 0.04 | |
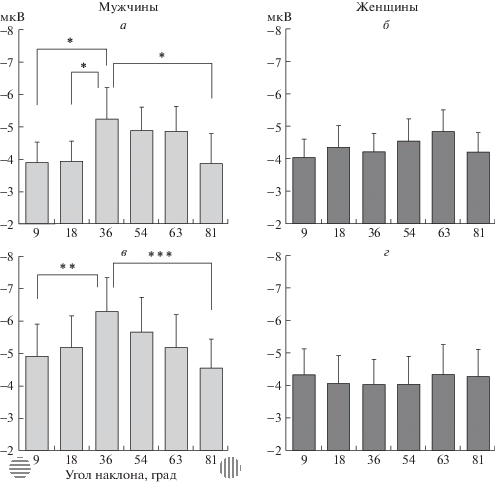
С учетом этих результатов был проведен последующий дисперсионный анализ амплитуды N1, усредненной по электродам затылочного полюса. Как и на предыдущем этапе анализа, в правом полушарии выявлено взаимодействие “ориентация” × “пол” (F5,160 = 4.39; p < 0.001). В левом полушарии выявлен эффект “ориентации” (F5,160 = 4.57; p < 0.005). Рисунок 3 демонстрирует половые различия в чувствительности компонента N1 ВП затылочного кластера к наклону ориентационных решеток. Приведены значения амплитуды N1, усредненные для ВП, зарегистрированных от затылочных электродов в левом и правом полушариях (слева №№ 65, 66, 70 и справа №№ 83, 84, 90 соответственно). Как видно, зависимость амплитуды N1 от наклона ориентационной решетки есть только в группе мужчин и отсутствует в группе женщин. В группе мужчин амплитуда N1 максимальна на решетку с наклоном линий 36°, близком к 45°. По мере приближения к кардинальным осям амплитуда N1 уменьшается. Post-hoc сопоставления выявили значимые различия в левом полушарии для пар 36 и 18° (p < 0.05), 36 и 9° (p < 0.05), 36 и 81° (p < 0.05); в правом полушарии различия более отчетливы и значимы для пар 36 и 9° (p < 0.01), 36 и 81° (p < < 0.0001).
Рис. 3.
Амплитуда компонента N1 ВП затылочного кластера при решении задачи определения близости наклонных ориентаций к референтным осям.
а, б – данные для левого полушария; в, г – данные для правого полушария. По вертикали – амплитуда N1 (мкВ), по горизонтали – угол наклона ориентации. На фрагменте в обозначены горизонтальный и вертикальный референты. Приведены средние по группам мужчин и женщин значения амплитуды N1 и ошибки среднего. Достоверность статистических различий: *** – р < 0.001, ** – р < 0.01, * – р < 0.05.
ОБСУЖДЕНИЕ
В настоящей работе проведена сравнительная оценка способности мужчин и женщин к восприятию наклонных линий и оценке их близости к базовым референтам – горизонтали и вертикали, а также к наклону 45°. Анализ психофизических показателей выявил уменьшение точности выполнения задачи у женщин при отсутствии различий времени реакции. Нейрофизиологические показатели сенсорного звена анализа ориентаций в зрительных областях коры высокозначимо различались у мужчин и женщин. Только в группе мужчин амплитуда ранней негативности N1 зависела от наклона линий, что говорит о чувствительности нейронов зрительной коры к ориентационным свойствам стимула в этой группе испытуемых.
Сведения о половых различиях выполнения зрительно-пространственных задач широко представлены в литературе, но в большей степени касаются сложных форм поведения. Хорошо известны различия в навигационном поведении. Так, мужчины значительно лучше ориентируются в незнакомой местности (Boone et al., 2019). Они лучше отслеживают начальную точку при прохождении сложного маршрута, что дает им преимущество при нахождении пути назад (Gagnon et al., 2018). Мужчины быстрее проходят виртуальный лабиринт, справляются с поиском скрытых меток и локаций, при их поиске используют более короткие расстояния (Boone et al., 2019; Gagnon et al., 2018). Так же мужчины лучше воспринимают навигационные инструкции, использующие направления и конкретные расстояния (Saucier et al., 2002). Преимуществом женщин является лучшее выполнение задач на запоминание положения объектов в окружающей среде, как в двухмерном, так и в трехмерном пространстве (Bocchi et al., 2018; Iachini et al., 2005). Женщины запоминают значительно больше меток на маршруте по сравнению с мужчинами (Yagi, Galea, 2019), а при прохождении лабиринта пользуются бóльшим количеством ранее пройденных маршрутов (Boone et al., 2019), что свидетельствует о бóльшем объеме зрительно-пространственной памяти. Более мягкие половые различия наблюдаются при навигации в знакомой среде или в незнакомой среде, богатой ориентирами (Nazareth et al., 2019), при наличии неограниченного времени на изучение ранее незнакомой окружающей среды, а также в задачах, где требуется определение кардинальных направлений (север, юг, запад, восток) (Harris et al., 2019). Наблюдаемые половые различия связывают с различиями в стратегиях выполнения навигационных задач. Мужчины преимущественно используют стратегию, основанную на сканировании пространства и последующим построением ментальной карты местности, в которой учитываются пространственная геометрия и метрические характеристики, в том числе евклидовы координаты (Gagnon et al., 2018). Женщины предпочтительно используют стратегию, основанную на запоминании ориентиров и определенного маршрута, которая опирается на последовательность поворотов и ориентиров (Harris et al., 2019; Nazareth et al., 2019). В ряде случаев половые различия в зрительно-пространственной деятельности объясняют различиями уровня пространственной тревожности. Женщины, как правило, сообщают о более высокой пространственной тревоге, о неуверенности при решении пространственных задач, что оказывает сильное влияние на успешность навигационного поведения (Gagnon et al., 2018). При ознакомлении с незнакомой местностью женщины часто возвращаются в одно и то же место, совершая там короткие остановки, чтобы убедиться в его безопасности (Boone et al., 2019).
Для понимания причин возникновения половых различий выполнения зрительно-пространственных задач интересны сведения о роли половых гормонов в формировании этих навыков. Известно, что в онтогенезе действие половых гормонов определяет морфогенез различных органов и образование связей. В обзоре (Handa, McGivern, 2014) обобщаются данные о влиянии половых гормонов на развитие вентрального и дорзального путей переработки зрительной информации и, в связи с этим, на особенности анализа информации об объектах и движении у представителей разного пола как у животных, так и у человека. У животных половые различия морфологии зрительной системы выявлены уже на уровне ранних зрительных зон. Так, у самцов крыс на 20% больше нейронов в моно- и бинокулярных полях первичной зрительной коры по сравнению с самками, что связывают с протективным влиянием андрогенов на процесс клеточной смерти (Nuñez et al., 2000). У самцов крыс выше острота зрения по Вернье (Seymoure, Juraska, 1997). У мужчин, по данным функциональной магнитно-резонансной томографии, больше размер первичной зрительной коры в левом полушарии (Brun et al., 2009). По данным литературы, затылочная кора является одной из областей мозга с высокой плотностью рецепторов к андрогенам (Nuñez et al., 2001).
С пре- и постнатальным влиянием андрогенов связывают бóльшую успешность мужчин при выполнении задач, связанных с прицеливанием (“targeting skills”), такими как метание, удары ногами по мячу, попадание в цель (Kimura, 1999). В литературе это отличие связывают с влиянием пре- и постнатального уровня тестостерона на развитие дорзального пути, в частности, верхнетеменной области, специализированной для зрительно-моторного управления (Hines et al., 2003). В свою очередь женщины опережают мужчин при выполнении зрительных задач, которые включают опознание объектов, их семантическое обозначение. Женщины демонстрируют бóльший объем памяти на реальные объекты (Herlitz et al., 1999). Половые различия не ограничиваются простым распознаванием. В работе (Kramer et al., 1997) показано, что девочки уже с пятилетнего возраста опережают мальчиков в задаче свободного называния объектов, т.е. лучше справляются с решением задач, связанных со способностью вентрального пути к семантической связи объектов со словами и категориями. Эти способности проявляются в ситуации имплицитного обучения, когда женщины лучше мужчин воспроизводят нерелевантную задаче информацию об объектах (McGivern et al., 1997).
Проведенный в настоящей работе анализ психофизических характеристик выявил выраженную зависимость точности и ВР оценки близости тестовой линии к референтам от угла ее наклона. Ориентации, удаленные от референтов, опознавались хуже, чем близкие к ним. При этом в диапазоне тестовых наклонных ориентаций от вертикали до 45° ошибочные оценки смещены в сторону вертикали, а в диапазоне ориентаций от горизонтали до 45° ошибочные оценки смещены в сторону наклонного референта. Половые различия проявлялись снижением точности выполнения задачи у женщин по сравнению с мужчинами, в большей степени для ориентаций, удаленных от референтов. Для времени реакции половые различия отсутствовали.
Для обсуждения полученных результатов представляется интересным привлечь современные сведения о механизмах оценки угла наклона линии: модель вероятностей Байеса и модель внутренней мультимодальной референтной системы (“multimodal reference frame”). Согласно модели вероятностей Байеса, мозг принимает статистическое решение на основании предшествующего знания и поступающей в настоящий момент сенсорной информации (Сущин, 2017). При этом, чем меньше зрительной информации о стимуле поступает в мозг, тем больше вклад предварительного знания, т.е. в случае оценки ориентации линий, это зрительный опыт о преобладании в окружающей среде горизонталей и вертикалей (Patten et al., 2017). Согласно этой модели, в нашем исследовании при длительности тестового стимула 100 мс ситуация соответствовала “недостатку” зрительной информации. В этом случае следовало бы ожидать смещения ошибочных оценок в сторону базовых референтных осей. Такое смещение мы наблюдали для тестовых ориентаций, расположенных в секторе от вертикали до 45°. Напротив, в секторе от горизонтали до 45° преобладали ошибки не в сторону горизонтали, а в сторону наклонного референта.
Полученные нами результаты в большей степени соответствуют классическим представлениям об определении наклона в координатах внутренней мультимодальной референтной системы. Эта система интегрирует информацию от вестибулярных, тактильных и проприорецепторов, а также периферической зрительной информации и формирует внутренние эталоны – субъективную зрительную вертикаль (СЗВ) и субъективную зрительную горизонталь (СЗГ) (Luyat et al., 2012; Alberts et al., 2016; Cuturi, Gori, 2019). Референтная система является пластичной и хорошо адаптируется к внешним условиям (Luyat et al., 2005; Alberts et al., 2016). Наше исследование проводилось в затемненном помещении, горизонтальные и вертикальные края монитора были закрыты экраном с круглой апертурой. Влияние дополнительной зрительной информации было минимизировано, и в этих условиях гравитационная составляющая оказывала значительное влияние на формирование внутренних эталонов (Dakin, Rosenberg, 2019). Доминирование СЗВ определило большую точность и меньшее ВР для определения линий, близких к вертикали, и преобладание “вертикальных” ошибочных ответов.
Ранее в работе (Mikhailova et al., 2018) мы публиковали сведения о половых различиях вызванных ответов различных областей коры при идентификации базовых и наклонных (45 и 135°) ориентаций. Статистически значимую зависимость от пола выявили только для ранних ответов (P100 и N150) каудальных областей коры, что можно рассматривать как проявление недостаточности раннего звена переработки информации об ориентационных характеристиках в группе женщин. Половые различия проявлялись в большем количестве ошибок идентификации наклонных ориентаций 45 и 135°, на что указывали и другие авторы (Ocklenburg et al., 2011). В настоящем исследовании определение близости к базовым осям не включало точную оценку тестовой ориентации. В этом случае отсутствие выраженных половых различий, как это было зафиксировано у этих же испытуемых в тесте Бентона (Славуцкая и др., 2014), неудивительно и сходно с результатами работы (Iachini et al., 2005), где оба пола успешно вспоминали расположение предметов друг относительно друга; мужчины опережали женщин только при воспроизведении метрических характеристик.
Нейрофизиологические характеристики раннего анализа наклонных ориентаций продемонстрировали отчетливые половые различия. Только в группе мужчин мы наблюдали зависимость амплитуды компонента N1 от наклона ориентаций. Наибольшую степень зависимости амплитуды ответа от ориентации линии наблюдали на электродах затылочной области с максимальной амплитудой N1 в ответах на ориентации, отдаленные от кардинальных осей и близкие к 45°.
Нейронный механизм оценки наклонных ориентаций пока недостаточно ясен. Лишь небольшое количество нейронов зрительной коры демонстрируют оптимальную ориентационную настройку на наклонные линии (Li et al., 2003). Единичные исследования с регистрацией ВП свидетельствуют о важности для идентификации наклонных ориентаций интервала компонента N1, который связывают с этапом сенсорной категоризации. По данным (Takács et al., 2013), в затылочных и теменно-затылочных отведениях амплитуда негативности во временном окне 120–190 мс коррелирует со степенью отклонения наклонных линий от кардинальных осей: чем больше отклонение, тем больше амплитуда N1. Связь N1 с анализом наклонных ориентаций подтверждается результатами работы (Song et al., 2010): при повторных тренировках улучшение идентификации наклонных линий приводит к снижению амплитуды N1 в ВП затылочно-теменных областей. Считается, что в механизме чувствительности нейронов затылочной коры к наклонным ориентациям важную роль играют нисходящие влияния со стороны более высоких уровней зрительного анализатора (Huang et al., 2006; Koelewijn et al., 2011).
О более сложном, не связанном с нейронами-детекторами, механизме чувствительности к наклонным ориентациям свидетельствуют данные экспериментальных работ и единичных исследований на человеке. Хорошо известна роль тормозных процессов в ориентационной настройке нейронов зрительной коры (Sillito, 1975). При обсуждении вопроса, как торможение, опосредованное ГАМК-интернейронами, может влиять на задачу дискриминации ориентаций, в литературе предполагается участие двух механизмов. Первый – обусловленное локальным торможением обострение ориентационной настройки (Sillito, 1975). Второй – кооперативное взаимодействие в сетях нейронов зрительных областей с предполагаемой ролью гамма-осцилляций как механизма их объединения (Samonds, Bonds, 2005). Для обсуждения механизма “oblique” эффекта интерес представляют данные исследования Эдден и соавт. (Edden et. al., 2009), направленного на анализ нейронных механизмов ориентационной чувствительности в зрительной коре человека. Опираясь на данные литературы о связи частоты гамма-осцилляций (30–80 Hz) с ориентационной настройкой (Friedman-Hill et al., 2000), а также с уровнем ГАМК в покое (Muthukumaraswamy et al., 2009), Эдден и соавт. поставили вопрос, существует ли в зрительной системе человека связь этих показателей с поведенческими проявлениями ориентационной чувствительности. Было установлено, что пороги дискриминации в диапазоне наклонных ориентаций в 5 раз выше по сравнению с порогами для ориентаций близких к вертикали. Причем значения пороговой чувствительности в диапазоне наклонных линий коррелировали с концентрацией ГАМК и частотой гамма-осцилляций в зрительной коре. Следовательно, у человека выполнение задач дискриминации наклонных ориентаций может быть связано с процессами в локальных нейросетях зрительной коры, где ключевую роль играют ГАМК-эргические гамма-осцилляции. В подтверждение этого можно привести факт положительной корреляции способности к дискриминации наклонных ориентаций с уровнем тормозного нейромедиатора ГАМК у людей с высокой представленностью аутистических черт (Dickinson et al., 2014).
В настоящее время нет убедительных доказательств существования половых различий в деятельности нейротрансмиттерных систем. Есть лишь единичные указания на роль стероидных гормонов в развитии некоторых нейротрансмиттер-опосредованных нервных цепей в ЦНС, что проявляется, например, в половом диморфизме некоторых психических заболеваний (Krolick et al., 2018). Можно думать, что одним из важных механизмов обнаруженных в настоящем исследовании половых различий переработки ориентационных свойств зрительной среды являются связанные с пре- и постнатальным влиянием половых гормонов особенности развития дорзального и вентрального путей переработки зрительной информации (Handa, McGivern, 2014). Они проявляются не только при выполнении сложных форм зрительного поведения, но и на уровне ранних перцептивных операций, среди которых важное место занимает ориентационная чувствительность. В настоящем исследовании показано, что ранние ориентационно-селективные ответы затылочной коры на наклонные линии выявляются только в группе мужчин. Сходные данные о половых различиях динамики ранней позитивности ВП теменной коры при повороте отдельных элементов фигуры приведены в нашей работе (Mikhailova et al., 2012). Можно предположить, что в зрительной системе возможность определения точных метрических характеристик, таких как длина, размер, угол наклона во многом определяются особенностями ее морфофункциональной организации, формирование которой в онтогенезе находится под влиянием гормональных факторов (Handa, McGivern, 2014). Важную роль играют связанные с полом особенности зрительно-вестибулярной интеграции, в частности, связи зрительных областей с парието-инсулярным комплексом (Barnett-Cowan et al., 2010). На возможность гендерной специфичности функциональных связей в зрительной системе (visual network) указывают данные сравнительного исследования организации нейросетей покоя у мужчин и женщин (Clemens et al., 2017).
ЗАКЛЮЧЕНИЕ
В работе исследовали половые различия психофизических и электроэнцефалографических характеристик выполнения задачи определения близости наклонных ориентаций к референтным осям (вертикаль, горизонталь, наклон 45°). Показано, что женщины совершают больше ошибок по сравнению с мужчинами, но не обнаруживают различий во времени реакции. Выявлены половые различия раннего анализа наклонных ориентаций. Только в группе мужчин амплитуда ранней негативности N1 ВП затылочной коры зависела от наклона линий: минимальные значения амплитуды характерны для ответа на наклонные линии, близкие к кардинальным осям, максимальные – для близких к 45°. Предполагается, что в основе половых различий ранней чувствительности затылочной коры к наклонным ориентациям лежат особенности переработки информации в дорзальном и вентральном зрительных путях, определяемые пре- и постнатальным влиянием стероидных гормонов (Handa, McGivern, 2014).
Исследование выполнено при финансовой поддержке РФФИ, проект № 19-013-00918 A.
Список литературы
Михайлова Е.С., Герасименко Н.Ю., Крылова М.А., Изъюров И.В., Славуцкая А.В. Механизмы ориентационной чувствительности зрительной системы человека. Сообщение II. Корковые механизмы ранних этапов переработки информации об ориентации линий. Физиология человека. 2015. Т. 41 (3). С. 5–18. https://doi.org/10.7868/S013116461503011X
Славуцкая А.В., Герасименко Н.Ю., Михайлова Е.С. Механизмы ориентационной чувствительности зрительной системы человека. Сообщение I. Поведенческие характеристики ориентационной чувствительности. Влияние характера задачи, экспериментальных условий и пола. Физиология человека. 2014. Т. 40 (6). С. 88–97. https://doi.org/10.7868/S0131164614050154
Сущин М.А. Байесовский разум: Новая перспектива в когнитивной науке. Вопросы философии. 2017. Т. 3. С. 74–87.
Alberts B.B.G.T., de Brouwer A.J., Selen L.P.J., Medendorp W.P. A Bayesian account of visual-vestibular interactions in the rod-and-frame task. 2016. Eneuro. V. 3 (5). P. 1–14. https://doi.org/10.1523/ENEURO.0093-16.2016
Appelle S. Perception and discrimination as a function of stimulus orientation: The “oblique effect” in man and animals. Psychological Bulletin. 1972. V. 78 (4). P. 266–278. https://doi.org/10.1037/h0033117
Barnett-Cowan M., Dyde R.T., Thompson C., Harris L.R. Multisensory determinants of orientation perception: task-specific sex differences. Europ. J. Neurosci. 2010. V. 31 (10). P. 1899–1907. https://doi.org/10.1111/j.1460-9568.2010.07199.x
Bloem I.M., Ling S. Attentional modulation interacts with orientation anisotropies in contrast perception. J. Vision. 2017. V. 17 (11). P. 1–14. https://doi.org/10.1167/17.11.6
Bocchi A., Palermo L., Boccia M., Palmiero M., D’Amico S., Piccardi L. Object recognition and location: Which component of object location memory for landmarks is affected by gender? Evidence from four to ten year-old children. Applied Neuropsychology: Child. 2018. P. 1–10. https://doi.org/10.1080/21622965.2018.1504218
Boone A.P., Maghen B., Hegarty M. Instructions matter: Individual differences in navigation strategy and ability. Memory and Cognition. 2019. V. 47 (7). P. 1401–1414. https://doi.org/10.3758/s13421-019-00941-5
Brun C.C., Leporé N., Luders E., Chou Y.Y., Madsen S.K., Toga A.W., Thompson P.M. Sex differences in brain structure in auditory and cingulate regions. NeuroReport. 2009. V. 20 (10). P. 930–935. https://doi.org/10.1097/WNR.0b013e32832c5e65
Butler T., Imperato-McGinley J., Pan H., Voyer D., Cordero J., Zhu Y.S., Stern E., Silbersweig D. Sex differences in mental rotation: Top-down versus bottom-up processing. NeuroImage. 2006. V. 32 (1). P. 445–456. https://doi.org/10.1016/j.neuroimage.2006.03.030
Campbell F.W., Kulikowski J.J. Orientational selectivity of the human visual system. J. Physiol. 1966. V. 187 (2). P. 437–445. https://doi.org/10.1113/jphysiol.1966.sp008101
Caparelli-Dáquer E.M., Oliveira-Souza R., Moreira Filho P.F. Judgment of line orientation depends on gender, education, and type of error. Brain and Cognition. 2009. V. 69 (1). P. 116–120. https://doi.org/10.1016/j.bandc.2008.06.001
Clemens B., Junger J., Pauly K., Neulen J., Neuschaefer-Rube C., Frölich D., Mingoia G., Derntl B., Habel U. Male-to-female gender dysphoria: Gender-specific differences in resting-state networks. Brain and Behavior. 2017. V. 7 (5). e00691–e00691. https://doi.org/10.1002/brb3.691
Collaer M.L., Nelson J.D. Large visuospatial sex difference in line judgment: Possible role of attentional factors. Brain and Cognition. 2002. V. 49 (1). P. 1–12. https://doi.org/10.1006/brcg.2001.1321
Cuturi L.F., Gori M. Biases in the visual and haptic subjective vertical reveal the role of proprioceptive/vestibular priors in child development. Frontiers in Neurology. 2019. V. 10. P. 1–10. https://doi.org/10.3389/fneur.2018.01151
Dakin C.J., Rosenberg A. Gravity estimation and verticality perception. Handbook of Clinical Neurology. 2019. V. 159. P. 43–59. https://doi.org/10.1016/B978-0-444-63916-5.00003-3
Dickinson A., Jones M., Milne E. Oblique orientation discrimination thresholds are superior in those with a high level of autistic traits. J. Autism and Developmental Disorders. 2014. V. 44. P. 2844–2850. https://doi.org/10.1007/s10803-014-2147-1
Dragoi V., Turcu C.M., Sur M. Stability of cortical responses and the statistics of natural scenes. Neuron. 2001. V. 32 (6). P. 1181–1192. https://doi.org/10.1016/S0896-6273
Edden R.A., Muthukumaraswamy S.D., Freeman T.C., Singh K.D. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 2009. V. 29 (50). P. 15721–15726. https://doi.org/10.1523/jneurosci.4426-09.2009
Friedman-Hill S., Maldonado P.E., Gray C.M. Dynamics of striate cortical activity in the alert macaque: I. Incidence and stimulus-dependence of gamma-band neuronal oscillations. Cerebral Cortex. 2000. V. 10 (11). P. 1105–1116. https://doi.org/10.1093/cercor/10.11.1105
Furmanski C., Engel S. An oblique effect in human primary visual cortex. Nature Neuroscience. 2000. V. 3. P. 535–536. https://doi.org/10.1038/75702
Gagnon K.T., Thomas B.J., Munion A., Creem-Regehr S.H., Cashdan E.A., Stefanucci J.K. Not all those who wander are lost: Spatial exploration patterns and their relationship to gender and spatial memory. Cognition. 2018.V. 180. P. 108–117. https://doi.org/10.1016/j.cognition.2018.06.020
Handa R.J., McGivern R.F. Steroid hormones, receptors, and perceptual and cognitive sex differences in the visual system. Current Eye Research. 2014. V. 40 (2). P. 110–127. https://doi.org/10.3109/02713683.2014.952826
Hansen B.C., Essock E.A. A horizontal bias in human visual processing of orientation and its correspondence to the structural components of natural scenes. J. Vision. 2004. V. 4 (12). P. 1044–1060. https://doi.org/10.1167/4.12.5
Harris T.A., Scheuringer A., Pletzer B. Perspective and strategy interactively modulate sex differences in a 3D navigation task. Biol. Sex Differences. 2019. V. 10 (1). P. 1–12. https://doi.org/10.1186/s13293-019-0232-z
Hines M., Fane B.A., Pasterski V.L., Mathews G.A., Conway G.S., Brook C. Spatial abilities following prenatal androgen abnormality: Targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003. V. 28 (8). P. 1010–1026. https://doi.org/10.1016/S0306-4530(02)00121-X
Herlitz A., Airaksinen E., Nordström E. Sex differences in episodic memory: The impact of verbal and visuospatial ability. Neuropsychology. 1999. V. 13 (4). P. 590–597. https://doi.org/10.1037/0894-4105.13.4.590
Huang L., Shou T., Yu H., Sun C., Liang Z. Slab-like functional architecture of higher order cortical area 21a showing oblique effect of orientation preference in the cat. NeuroImage. 2006. V. 32. P. 1365–1374. https://doi.org/10.1016/j.neuroimage.2006.05.007
Hubel D.H., Wiesel T.N. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiology. 1962. V. 160 (1). P. 106–154. https://doi.org/10.1113/jphysiol.1962.sp006837
Iachini T., Sergi I., Ruggiero G., Gnisci A. Gender differences in object location memory in a real three-dimensional environment. Brain and Cognition. 2005. V. 59 (1). P. 52–59. https://doi.org/10.1016/j.bandc.2005.04.004
Kimura D. Sex and cognition. Cambridge, MA: MIT Press, 1999. 230 p. ISBN: 9780262112369
Koelewijn L., Dumont J.R., Muthukumaraswamy S.D., Rich A.N., Singh K.D. Induced and evoked neural correlates of orientation selectivity in human visual cortex. NeuroImage. 2011. V. 54 (4). P. 2983–2993. https://doi.org/10.1016/j.neuroimage.2010.11.045
Kramer J.H., Kaplan E., Delis D.C., O’Donnell L., Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997. V. 11 (4). P. 577–584. https://doi.org/10.1037/0894-4105.11.4.577
Krolick K.N., Zhu Q., Shi H. Effects of estrogens on central nervous system neurotransmission: Implications for sex differences in mental disorders. Prog. Molecular Biol. Translational Sci. 2018. V. 160. P. 105–171. https://doi.org/10.1016/bs.pmbts.2018.07.008
Lee J., Lee C. Changes in orientation discrimination at the time of saccadic eye movements. Vision Research. 2008. V. 48 (21). P. 2213–2223. https://doi.org/10.1016/j.visres.2008.06.014
Li B., Peterson, M.R., Freeman R.D. Oblique effect: A neural basis in the visual cortex. J. Neurophysiol. 2003. V. 90 (1). P. 204–217. https://doi.org/10.1152/jn.00954.2002
Luyat M., Mobarek S., Leconte C., Gentaz E. The plasticity of gravitational reference frame and the subjective vertical: Peripheral visual information affects the oblique effect. Neuroscience Letters. 2005. V. 385 (3). P. 215–219. https://doi.org/10.1016/j.neulet.2005.05.044
Luyat M., Noël M., Thery V., Gentaz E. Gender and line size factors modulate the deviations of the subjective visual vertical induced by head tilt. BMC Neuroscience. 2012. V. 13 (1). P. 1–8. https://doi.org/10.1186/1471-2202-13-28
McGivern R.F., Huston J.P., Byrd D., King T., Siegle G.J., Reilly J. Sex differences in visual recognition memory: Support for a sex-related difference in attention in adults and children. Brain and Cognition. 1997. V. 34 (3). P. 323–336. https://doi.org/10.1006/brcg.1997.0872
Mikhailova E.S., Gerasimenko N.Y., Slavutskaya A.V. Effects of line orientation in visual evoked potentials. Spatial dynamics. and gender differences of neural oblique effect. BioRxiv. 2018. 323782. https://doi.org/10.1101/323782
Mikhailova E.S., Slavutskaya A.V., Gerasimenko N.Yu. Gender differences in the recognition of spatially transformed figures: Behavioral data and event-related potentials (ERPs). Neuroscience Letters. 2012. V. 524 (2). P. 74–78.
Muthukumaraswamy S.D., Edden R.A.E., Jones D.K., Swettenham J.B., Singh K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. National Acad. Sci. 2009. V. 106 (20). P. 8356–8361. https://doi.org/10.1073/pnas.0900728106
Nasr S., Tootell R.B. Role of fusiform and anterior temporal cortical areas in facial recognition. Neuroimage. 2012. V. 63 (3). P. 1743–1753. https://doi.org/10.1016/j.neuroimage.2012.08.031
Nazareth A., Huang X., Voyer D., Newcombe N. A meta-analysis of sex differences in human navigation skills. Psychonomic Bull. Rev. 2019. V. 26 (5). P. 1503–1528. https://doi.org/10.3758/s13423-019-01633-6
Nuñez J.L., Jurgens H.A., Juraska J.M. Androgens reduce cell death in the developing rat visual cortex. Developmental Brain Research. 2000. V. 125 (1–2). P. 83–88. https://doi.org/10.1016/S0165-3806
Nuñez J.L., Lauschke D.M., Juraska J.M. Cell death in the development of the posterior cortex in male and female rats. J. Comparative Neurol. 2001. V. 436 (1). P. 32–41. https://doi.org/10.1002/cne.1051
Ocklenburg S., Hirnstein M., Ohmann H.A., Hausmann M. Mental rotation does not account for sex differences in left-right confusion. Brain and Cognition. 2011. V. 76 (1). P. 166–171. https://doi.org/10.1016/j.bandc.2011.01.010
Patten M.L., Mannion D.J., Clifford C.W.G. Correlates of perceptual orientation biases in human primary visual cortex. J. Neurosci. 2017. V. 37 (18). P. 4744–4750. https://doi.org/10.1523/JNEUROSCI.3511-16.2017
Proverbio A.M., Esposito P., Zani A. Early involvement of the temporal area in attentional selection of grating orientation: an ERP study. Cognitive Brain Research. 2002. V. 13 (1). P. 139–151. https://doi.org/10.1016/s0926-6410(01)00103-3
Samonds J., Bonds A.B. Gamma Oscillation maintains stimulus structure-dependent synchronization in cat visual cortex. J. Neurophysiol. 2005. V. 93. P. 223-36. https://doi.org/10.1152/jn.00548.2004
Saucier D.M., Green S.M., Leason J., MacFadden A., Bell S., Elias L.J. Are sex differences in navigation caused by sexually dimorphic strategies or by differences in the ability to use the strategies? Behavioral Neuroscience. 2002. V. 116 (3). P. 403–410. https://doi.org/10.1037/0735-7044.116.3.403
Seymoure P., Juraska J.M. Vernier and grating acuity in adult hooded rats: The influence of sex. Behavioral Neuroscience. 1997. V. 111 (4). P. 792–800. https://doi.org/10.1037/0735-7044.111.4.792
Shaqiri A., Roinishvili M., Grzeczkowski L., Chkonia E., Pilz K., Mohr C., Brand A., Kunchulia M., Herzog M.H. Sex-related differences in vision are heterogeneous. Scientific reports. 2018. V. 8 (1). P. 7521. https://doi.org/10.1038/s41598-018-25298-8
Sillito A.M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. The J. Physiol. 1975. V. 250 (2). P. 305–329. https://doi.org/10.1113/jphysiol.1975.sp011056
Song Y., Sun L., Wang Y., Zhang X., Kang J., Ma X., Yang B., Guan Y., Ding Y. The effect of short-term training on cardinal and oblique orientation discrimination: An ERP study. Intern. J. Psychophysiol. 2010. V. 75 (3). P. 241–248. https://doi.org/10.1016/j.ijpsycho.2009.11.007
Takács E., Sulykos I. Czigler I., Barkaszi. I., Balázs L. Oblique effect in visual mismatch negativity. Frontiers in Human Neuroscience. 2013. V. 7. P. 591. https://doi.org/10.3389/fnhum.2013.00591
Yagi S., Galea L.A.M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology. 2018. V. 44 (1). P. 200–213. https://doi.org/10.1038/s41386-018-0208-4
Yang B., Ma X., Schweinhart A.M., Wang F., Sun M., Song Y. Comparison of event-related potentials elicited by cardinal and oblique orientations with broad-band noise stimuli. Vision Research. 2012. V. 60. P. 95–100. https://doi.org/10.1016/j.visres.2012.03.011
Yashar A., Denison R.N. Feature reliability determines specificity and transfer of perceptual learning in orientation search. PLOS Computational Biology. 2017. V. 13 (12). e1005882. https://doi.org/10.1371/journal.pcbi.1005882
Дополнительные материалы отсутствуют.
Инструменты
Сенсорные системы


