Зоологический журнал, 2019, T. 98, № 4, стр. 393-406
A New Species of the Genus Bembecia Hübner 1819 [“1816”] from the European Part of Russia (Lepidoptera, Sesiidae), with Remarks on the Bembecia dispar (Staudinger 1891) Species Group
O. G. Gorbunov* *
Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: gorbunov.oleg@mail.ru
Поступила в редакцию 13.07.2018
После доработки 8.08.2018
Принята к публикации 7.09.2018
Аннотация
A new species, Bembecia rossica sp. n., is described and illustrated from the Astrakhan Region of Russia. Data on its habitat, host plant and larval biology are presented and illustrated as well. Bembecia tsvetajevi O. Gorbunov 1992 stat. rev. is resurrected from synonymy under B. polyzona (Püngeler 1912), while B. kappadocica Špatenka 1997 stat. n. is treated as a distinct species, but not as a subspecies of B. syzcjovi O. Gorbunov 1991.
The palaearctic genus Bembecia Hübner 1819 [“1816”] is the largest sesiid genus of the tribe Synanthedonini. Bembecia currently consists of 119 species (Gorbunov, Efetov, 2018; Gorbunov, 2018), but one may expect a noticeable increase in this number due to continuous discoveries of new species from different parts of the Palaearctic, especially in its southern areas (Gorbunov et al., 2017).
So far, only 16 species of the genus have been known to occur in Russia (Gorbunov, 2008; Efetov et al., 2012; Gorbunov, 2018; Gorbunov, Efetov, 2018). Below I describe a new species, B. rossica sp. n., thus bringing the total diversity of Bembecia species in Russia to 17.
By the structure of both male and female genitalia, the genus can be divided into three subgenera: Bembecia s. str., Opacosphecia Căpuşe 1973, and Scalarignathia Căpuşe 1973 (Gorbunov, Efetov, 2018). Besides this, the former two subgenera can be split into several species groups. I place the new species to the B. dispar (Staudinger 1891) species group of the nominative subgenus.
In the process of preparing this work, I had to carry out a serious work with finding out the taxonomic position of the following four taxa: B. polyzona (Püngeler 1912) (type locality: “Trancaspia, Merv” [= Turkmenistan: Mary), B. tsvetajevi O. Gorbunov 1992 (type locality: Turkmenistan: Ashkhabad), B. syzcjovi Gorbunov 1991 (type locality: Georgia: Meskhetian Mts), and B. syzcjovi kappadocica Špatenka 1997 (type locality: Turkey: Cappadocia, Yeşilöz).
B. polyzona was described from four syntypes (Püngeler, 1912) which turned out to represent two different species (Kallies, Bartsch, 2010). They were collected in two different places: one female originated from “Trancaspia, Merv”, while two males and one female were collected in “Central Asia, Ilidistrict” (Püngeler, 1912: 394). The female from Merv was selected and fixed as the lectotype by Căpuşe (1973: 144). In their publication, Kallies and Bartsch (2010), absolutely without any argument, claimed that “The female specimen from Merw (Turkmenistan) which Căpuşe (1973) selected as lectotype (Fig. 6), is conspecific with Bembecia tsvetajevi Gorbunov 1992, which thus becomes a junior subjective synonym of B. polyzona” (Kallies, Bartsch, 2010: 83–84). I do not agree with this statement. First of all, the female of B. polyzona (unfortunately, the male is still unknown) differs from the yellow form of the female of B. tsvetajevi (Figs 1c, 1d) by the colouration of the abdomen (tergite 4 dark brown with greenish sheen, tergites 2, 3, 5 and 6 each with a broad yellow stripe distally in B. polyzona, vs. tergite 3 dark brown to black with dark bronze sheen, tergite 2 with a broad yellow stripe distally and tergites 4–6 each yellow with a narrow dark brown stripe proximally in B. tsvetajevi) and the discal spot of the forewing (distal two-thirds orange-yellow in B. polyzona, vs. with a few yellow scales distally in B. tsvetajevi), as well as by the conformation of the transparent area of the forewing (anterior transparent area small, external transparent area divided into five cells, level to vein M2 about 1.3 times as broad as discal spot in B. polyzona, vs. transparent area completely covered with yellow scales in B. tsvetajevi). In addition, by the structure of the female genitalia (see fig. 4 in Căpuşe, 1973), B. polyzona seems to belong to the B. senilis (Groum-Grshimailo 1890) species group. Possibly the geographical label of the lectotype (“Trancaspia, Merv”) is false and in fact the specimen comes from somewhere in the mountains of the eastern part of Central Asia. Besides this, the host plant of B. tsvetajevi is highly likely to be Astragalus schahrudensis Bunge (Fabaceae), which is a mountainous species and does not occur in the flood-plain of the Murgab River in the southern part of the Kara-Kum Desert. In short, B. tsvetajevi O. Gorbunov 1992 stat. rev. (Figs 1a–1f), which was synonymized with B. polyzona (Püngeler 1912) by Kallies and Bartsch (2010), is here considered as full valid species.
Fig. 1.
Bembecia tsvetajevi O. Gorbunov 1992. a – male, paratype, upside. Turkmenistan, Ashkhabad, 15.08.1928, P. Donov leg. (COGM). Sesiidae picture № 0229–2018. Alar expanse 27.0 mm, b – ditto, underside. Sesiidae Picture № 0230–2018; c – female, paratype, upside. Turkmenistan, Ashkhabad, 15.08.1928, P. Donov leg. (COGM). Sesiidae picture № 0231–2018. Alar expanse 21.5 mm, d – ditto, underside. Sesiidae Picture № 0232–2018; e – female, paratype, upside. Turkmenistan, Ashkhabad, 15.08.1928, P. Donov leg. (ZMMU). Sesiidae picture № 0455–2016. Alar expanse 25.0 mm, f – ditto, underside. Sesiidae Picture № 0456–2016.

Fig. 2.
Bembecia spp. (a–d): B. syzcjovi O. Gorbunov 1991. a – male, holotype, upside. Georgia, 20 km NE of Akhaltsykhe, 12.07.1988, ex. l., O. Gorbunov leg. (COGM). Sesiidae picture № 0233–2018. Alar expanse 24.9 mm, b – ditto, underside. Sesiidae Picture № 0234–2018; c – male, paratype, upside. Georgia, 20 km NE of Akhaltsykhe, 12.07.1988, ex. l., O. Gorbunov leg. (COGM). Sesiidae picture № 0235–2018. Alar expanse 24.0 mm, d – ditto, underside. Sesiidae Picture № 0236–2018. (e–h): B. kappadocica Špatenka 1997 stat. n. e – male, paratype, upside. Turkei, Kappadocia, Yeşilöz, 7.06.1993, K. Špatenka leg. (COGM). Sesiidae picture № 0241–2018. Alar expanse 25.5 mm, f – ditto, underside. Sesiidae Picture № 0242–2018; g – female, paratype, upside. Turkei, Kappadocia, Yeşilöz, 7.06.1993, K. Špatenka leg. (COGM). Sesiidae picture № 0243–2018. Alar expanse 26.0 mm, h – ditto, underside. Sesiidae Picture № 0244–2018.

In his description of B. syzcjovi kappadocica, Špatenka (1997a) gave neither a differential diagnosis nor pictures of both male and female genitalia. Having carefully studied the external morphology, as well as the structure of the male genitalia, I come to the conclusion that B. syzcjovi kappadocica is not a subspecies of B. syzcjovi (Figs 2a–2d), but a distinct species, B. kappadocica Špatenka 1997 stat. n. (Figs 2e–2h).
The material studied, including the holotype and most of the paratypes of the new species, is kept in the collection of the A.N. Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia (COGM). Part of the paratypes of B. rossica sp. n. will be transferred to the collections of the Zoological Institute of the Russian Academy of Sciences, St.-Petersburg, Russia (ZISP) and the Zoological Museum of the M.V. Lomonosov Moscow State University, Moscow, Russia (ZMMU).
All labels of the holotype of B. rossica sp. n. are cited verbatim, where each label is separated by quotation marks; lines in a label are separated by a slash (“/”).The labels of geographical data, imaging data and genitalia preparation numbers are printed on white paper, but the determination labels of the types are printed on red paper.
Images of the habitats and host plant were taken using a Minolta® Dynax 7000i camera equipped with a Minolta® 50 f/2.8 Macro lens on a Kodak® Ectochrome® 100VS film and then scanned with a Nikon® LS-2000 slide scanner. The images of the moths were taken with a Sony® α450 DSLR camera equipped with a Minolta® 50 f/2.8 Macro lens. The genitalia were photographed using a Keyence® BZ-9000 Biorevo Fluorescence Microscope. The processing of all illustrations was finalized with Adobe® Photoshop® CS5 software.
All illustrated dry specimens and their pictures are labeled with a number containing the name of the family, two consecutive digits and a year (e.g. SESIIDAE pictures № 0283-0284–2013). These numbers correspond to those of the figured specimens in the author’s archive.
Genital preparations are kept in microtubes pinned under the specimen. All genitals have the appropriate number placed inside the microtube. This number as a label (e.g. Genital preparation № OG–011-2014) is pinned under the specimen and listed in the author’s archive.
Bembecia (s. str.) rossica O. Gorbunov sp. n.
(Figs 3–5, 8)
Fig. 3.
Variability of males of Bembecia rossica sp. n. a – holotype, upside. Sesiidae picture № 0283–2013. Alar expanse 25.2 mm, b – ditto, underside. Sesiidae Picture № 0284–2013; c – paratype, upside. Sesiidae picture № 0279–2013. Alar expanse 27.0 mm, d – ditto, underside. Sesiidae picture № 0280–2013; e – paratype, upside. Sesiidae picture № 0277–2013. Alar expanse 27.0 mm, f – ditto, underside. Sesiidae picture № 0278–2013; g – paratype, upside. Sesiidae picture № 0273–2013. Alar expanse 23.9 mm, h – ditto, underside. Sesiidae picture № 0274–2013.

Fig. 4.
Variability of females of Bembecia rossica sp. n. a – paratype, upside. Sesiidae picture № 0267–2013. Alar expanse 27.0 mm. b – ditto, underside. Sesiidae picture № 0268–2013; c – paratype, upside. Sesiidae picture № 0265–2013. Alar expanse 28.0 mm. d – ditto, underside. Sesiidae picture № 0266–2013; e – paratype, upside. Sesiidae picture № 0263–2013. Alar expanse 26.2 mm. f – ditto, underside. Sesiidae picture № 0264–2013; g – paratype, upside. Sesiidae picture № 0269–2013. Alar expanse 27.5 mm. h – ditto, underside. Sesiidae picture № 0270–2013.

Fig. 5.
Genitalia of Bembecia rossica sp. n.: (a–e) – male, paratype, Genital preparation № OG–005-2015; (f) – female, paratype. Genital preparation № OG–006-2015. a – tegumen-uncus complex. b – valva. c – saccus. d – aedeagus. e – vesica. Scale bar (mm): a–d, f – 0.5, e – 0.2.

Fig. 6.
Variability of Bembecia deserticola Špatenka, Petersen & Kallies 1997. a – male, upside. Kazakhstan, Almaty Region, Lepsy River, 46°59′ N, 79°42′ E, 12.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0335–2013; Alar expanse 28.0 mm. b – ditto, underside. Sesiidae picture № 0336–2013; c – male, upside. Kazakhstan, Almaty Region, Lepsy River, 46°59′ N, 79°42′ E, 12.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0333–2013; Alar expanse 25.0 mm. d – ditto, underside. Sesiidae picture № 0334–2013; e – female, upside. Kazakhstan, Almaty Region, Lepsy River, 46°59′ N, 79°42′ E, 12.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0285–2015; Alar expanse 25.0 mm. f – ditto, underside. Sesiidae picture № 0286–2015; g – female, upside. Kazakhstan, Almaty Region, Lepsy River, 46°59′ N, 79°42′ E, 12.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0287–2015; Alar expanse 17.0 mm. h – ditto, underside. Sesiidae picture № 0288–2015.

Material. Holotype ♂ (Figs 3a, 3b) with labels: “Russia, Astrakhan Region, / 5 km N of Dosang, / –20 m, 46°56′ N, 47° 54′ E, / 20.VIII.2013, ex. p., / O. Gorbunov leg.”, “Host plant: / Astragalus vulpinus / (Fabaceae) / Moth emerged 05.IX.2013”, “Sesiidae / Pictures № / 0283-0284–2013 / Photo by O. Gorbunov”, “HOLOTYPUS ♂ / Bembecia rossica / O. Gorbunov, 2018 / O. Gorbunov des., 2017”. Paratypes (60 ♂♂, 60 ♀♀): 4 ♂♂, same locality, 10.06.2002, ex larvae from roots of Astragalus vulpinus (Fabaceae), moths emerged 29.08.–03.09.2003, leg. O. Gorbunov; 15 ♂♂, 18 ♀♀, same locality, 13.VIII.2003, ex larvae and pupae from roots of Astragalus vulpinus (Fabaceae), moths emerged 26.08.–20.09.2003, leg. O. Gorbunov (Sesiidae pictures № 0417-0420–2015) (1 ♂ with genitalia preparation № OG–005-2015); 3 ♂♂, 1 ♀, same locality, 04.VIII.2005, ex larvae and pupae from roots of Astragalus vulpinus (Fabaceae), moths emerged 21.08.–04.09.2005, leg. O. Gorbunov; 23 ♂♂, 21 ♀♀, same locality, 08.08.2007, ex larvae and pupae from roots of Astragalus vulpinus (Fabaceae), moths emerged 21.08.–16.09.2007, leg. O. Gorbunov; 15 ♂♂, 20 ♀♀, same locality and date as holotype, ex pupae from roots of Astragalus vulpinus (Fabaceae), moths emerged 20.08.–07.09.2013, leg. O. Gorbunov (Sesiidae pictures № 0261-0282–2013) (1 ♀ with genitalia preparation № OG–006-2015).
Description. Male (holotype) (Figs 3a, 3b). Alar expanse 25.2 mm; body length 17.0 mm; forewing 11.6 mm; antenna 8.0 mm. Head: antenna orange with apical quarter black with dark blue sheen; scapus black dorsally, yellow ventrally; frons yellow with golden sheen and a narrow pale yellow stripe laterally; vertex black densely mixed with pale yellow hairs; apical joint of labial palpus yellow with golden sheen, basal and mid joints each pale yellow dorsally, yellow ventrally with golden sheen and a narrow black stripe externally; occipital fringe pale yellow dorsally and laterally white. Thorax: patagia black dorsally with bronze-purple sheen and a few yellow scales with golden sheen, yellow with golden sheen laterally; tegula black with greenish-bronze sheen and an admixture of yellow scales, and with a large yellow spot with golden sheen at base of forewing; meso- and metathorax black with greenish-bronze sheen and a few yellow scales; besides this, tegula, meso- and metathorax densely covered with white hairs; thorax laterally dark brown to black with bronze-violet sheen and a large yellow spot with golden sheen anteriorly; both metepimeron and metameron posteriorly black with dark blue sheen densely covered with long white hairs. Legs: neck plate yellow with golden sheen; fore coxa yellow with golden sheen and an admixture of black scales; fore femur yellow with golden sheen dorsally, white ventrally; fore tibia dorsally yellow with golden sheen and a black base, ventrally white; fore tarsus entirely yellow with golden sheen and black spines ventrally; mid coxa black with greenish bronze sheen and a few yellow scales; mid femur internally pale yellow, externally black with dark blue-violet sheen posteriorly and pale yellow anteriorly; mid tibia internally pale yellow, externally yellow with golden sheen and a black spot with blue-violet sheen both basally and distally; spurs yellow to pale yellow with golden sheen; mid tarsus entirely yellow with golden sheen and black spines ventrally; hind coxa black with greenish-bronze sheen and an admixture of yellow scales; hind femur internally pale yellow, externally black with dark blue-violet sheen and a few yellow scales and dense admixture of white to pale yellow hairs; hind tibia internally pale yellow, externally yellow with golden sheen and a black spot with blue-violet sheen both basally and distally; spurs yellow to pale yellow with golden sheen; basal joint of hind tarsus pale yellow with golden sheen and black spines ventrally, remaining joints entirely yellow with golden sheen and black spines ventrally. Abdomen: tergite 1 dorsally black with greenish-blue sheen; tergite 2 black with greenish-blue sheen proximally and yellow with golden sheen medially and with a row of pale yellow scales with golden sheen distally; tergites 3 and 5 each narrowly black with greenish blue sheen proximally, yellow with golden sheen medially and narrowly black with strong violet-blue sheen distally; tergites 4 and 6 each yellow with golden sheen and a row of pale yellow scales with golden sheen distally; tergite 7 yellow with golden sheen and a row of black scales with strong violet-blue sheen distally; sternite 1+2 ventrally black with greenish blue sheen proximally and yellow with golden sheen distally; all other sternites yellow with golden sheen and a small, black, triangular spot with violet-blue sheen lateroproximally; anal tuft dorsally yellow to yellow-orange with golden sheen and two narrow black stripes with violet-blue sheen at margins of medial lobe; ventrally yellow-orange with golden sheen and two small black spots basally. Forewing: dorsally with basal part black with dark blue-violet sheen; costal margin up to tip of vein R1 brown, densely covered with yellow scales masking the background colouration; surface between veins R1–R3 brown to dark brown with dark purple-bronze sheen; anal margin yellow with a few brown scales distally; CuA-stem brown to dark brown with a few yellow scales; discal spot dark brown to black with dark blue-violet sheen and a very small and narrow yellow spot distally; common stem R4–5 and veins R4, R5, M3, CuA1, and CuA2 yellow, but densely covered with dark brown scales masking the background colouration; veins M1 and M2 yellow with a few brown scales distally; apical area yellow with a very narrow brown to dark brown outer margin with bronze-violet sheen; costal margin ventrally up to tip of vein R1 and CuA-stem pale yellow with golden sheen; anal margin, apical area and veins R2–CuA2 yellow with golden sheen; discal spot black with dark bronze-purple sheen and with a yellow-orange distal third; transparent areas well-developed, but densely covered with translucent scales with light golden-greenish hue; external transparent area large, divided into five cells between veins R3 and CuA1, level to vein M2 about thrice as broad as discal spot and about 3.7 times broader than apical area; cilia brown with bronze sheen. Hindwing: transparent; dorsally veins Sc, M1, M2, CuP, 1A and 2A yellow, other veins dark brown to black with dark bronze-purple sheen and a few yellow scales; discal spot narrow, cuneiform, reaching the base of common stem M3–CuA1, yellow with a few black scales; outer margin narrow, about 0.5 times as broad as cilia, between veins M1 and CuP dark brown to black with bronze sheen distally and yellow with golden sheen proximally, yellow-orange with golden sheen proximally of vein CuP; ventrally common stem M3–CuA1 and veins M3, CuA1 and CuA2 pale yellow with golden sheen, other veins yellow; discal spot yellow with a few dark brown scales; outer margin yellow with golden sheen and a very narrow dark brown to black distal margin between veins M1 and CuP; cilia brown to dark brown with bronze sheen.
Genitalia (paratype) (Genital preparation № OG–005-2015) (Figs 5a–5e). Tegumen-uncus complex relatively narrow; scopula androconialis well-developed, about 0.6 times as long as tegumen-uncus complex (Fig. 5a); crista gnathi lateralis narrow, slightly broadened and rounded distally; crista gnathi medialis undeveloped; (Fig. 5a); valva (Fig. 5b) trapeziform-ovoid, crista sacculi oblique, narrow, with a row of strong pointed setae anteriorly and a group of flat-topped ones posteriorly; saccus (Fig. 5c) relatively narrow, rounded basally, slightly longer than vinculum; aedeagus (Fig. 5d) thin, slightly bisinuate, about as long as valva; vesica with numerous small cornuti (Fig. 5e).
Female (paratype) (Figs 4a, 4b). Alar expanse 27.0 mm; body length 16.5 mm; forewing 12.1 mm; antenna 7.6 mm. Head: antenna orange with apical quarter black with dark blue sheen; scapus yellow-orange to orange with an admixture of individual black scales dorsally; frons and vertex yellow to yellow-orange with golden sheen; labial palpus yellow-orange ventrally and pale yellow dorsally; occipital fringe pale yellow dorsally and white laterally. Thorax: patagia yellow with golden sheen; tegula yellow-orange with golden sheen and a triangular black spot with greenish-violet sheen; mesothorax black with violet sheen densely covered with orange scales; metathorax yellow with golden sheen and a tuft of orange hairs laterally; thorax laterally entirely yellow-orange with golden sheen; both metepimeron and metameron posteriorly black with violet sheen and densely covered with orange hairs. Legs: fore leg completely yellow to yellow-orange with golden sheen; both mid and hind coxae black with blue-violet sheen; both mid and hind femurae externally yellow-orange with golden sheen and an admixture of black scales with blue-violet sheen; both mid and hind tibiae orange with golden sheen and a few black scales with blue-violet sheen both basally and at base of apical spurs; all spurs yellow-orange with golden sheen; both mid and hind tarsi yellow orange with golden sheen. Abdomen: tergite 1 entirely yellow-orange to orange with golden sheen dorsally; tergites 2, 4–6 each yellow-orange to orange with golden sheen and a narrow black stripe with dark greenish sheen proximally; tergite 3 yellow-orange to orange with golden sheen distally and black with dark greenish sheen proximally; ventrally orange with golden sheen and an admixture of black scales with dark violet sheen on sternite 1+2 and 3 proximally, and lateroproximally on sternites 4–6; anal tuft yellow-orange to orange with golden sheen and a narrow black stripe with violet-blue sheen on lateral lobe internally. Forewing: costal margin and CuA-stem dorsally orange with golden sheen and an admixture of black scales with blue-violet sheen; anal margin orange with golden sheen; discal spot black with dark violet sheen and orange proximal and distal margins; common stem R4–5 and veins M1 and M2 within external transparent area orange, veins R4, R5, M3, CuA1 and CuA2 and distal parts of veins M1 and M2 dark brown to black with blue-violet sheen; apical area yellow-orange to orange with golden sheen and a very narrow brown to dark brown outer margin with bronze-violet sheen; costal margin ventrally up to tip of vein R2 pale yellow with golden sheen; CuA-stem, anal margin, apical area and veins R2–CuA2 orange with golden sheen; discal spot orange with a narrow black spot with dark violet sheen subposteriorly; transparent areas well-developed, but densely covered with translucent scales with light golden-greenish hue; posterior transparent area not reaching the discal spot; external transparent area large, divided into five cells between veins R3 and CuA1, level to vein M2 about 2.3 times as broad as discal spot and about 3.5 times broader than apical area; cilia brown with bronze sheen. Hindwing: transparent; dorsally veins orange with golden sheen and an admixture of dark brown scales with dark violet sheen on veins CuA1 and CuA2; discal spot narrow, cuneiform, reaching the base of common stem M3–CuA1, orange; outer margin narrow, about 0.5 times as broad as cilia, between veins M1 and 1A dark brown to black with bronze sheen distally and yellow with golden sheen proximally, orange with golden sheen proximally of vein 1A; veins, discal spot and outer margin ventrally orange with golden sheen; cilia brown to dark brown with bronze sheen.
Genitalia (paratype) (Genital preparation № OG–006-2015) (Fig. 5f). Papillae anales relatively narrow and short, covered with short and long setae; tergite 8 broad with long setae ventrally and distally, triangular, with ventral margin folded inside; posterior apophysis about as long as anterior apophysis; both lamellae antevaginalis and postvaginalis undeveloped; ostium bursae slightly funnel-shaped, situated somewhat cranially than mid of tergite 8; antrum relatively narrow, short, about twice as short as anterior apophysis, well-sclerotized caudally and membranous cranially; ductus bursae membranous, slightly broader than antrum, with numerous wrinkles medially; short, about half as long as antrum; corpus bursae ovoid, without signum.
Individual variability. Both males (Figs 3a–3h) and females (Figs 4a–4h) vary in the number of yellow-orange and orange scales on the abdomen and hindwing. There is a male (Figs 3g, 3h), in which all yellow-orange scales on the thorax, legs, abdomen and wings are replaced with orange ones. Beside this, the transparent areas of the forewing are slightly variable as well. Individual size is variable as follows. Males: alar expanse 23.9–27.0 mm; body length 15.2–18.0 mm; forewing 11.0–12.5 mm; antenna 7.7–8.2 mm. Females: alar expanse 26.2–28.0 mm; body length 17.0–18.0 mm; forewing 12.9–12.9 mm; antenna 7.3–8.0 mm.
Differential diagnosis. This new species clearly belongs to the B. dispar (Staudinger 1891) ecological species group (partly as the “dispar-subgroup” in Špatenka et al., 1999). The main feature of most species in this ecological group is that their adult larvae have aestivation before pupation by late summer. Beside this, larvae of the species, for which the host plant is known, all live in roots of large herbaceous Astragalus spp. such as A. akkensis Coss., A. alopecurus Pall., A. gombo Bunge, A. ponticus Pall., A. vulpinus Willd., etc. (Fabaceae).
I include the following eight species into this ecological group, and they can be grouped into three different species groups by the structure of the male genitalia:
1) The B. dispar species-group with the following five species: B. dispar (Staudinger 1891) (type locality: Algeria: Biskra), B. tsvetajevi O. Gorbunov 1992 (type locality: Turkmenistan: Ashkhabad), B. deserticola Špatenka, Petersen et Kallies 1997 (type locality: Kazakhstan: Chu-Iliyskiy Mts., Kurdai Pass), B. pashtuna Špatenka 1997 (type locality: Pakistan, Baluchistan, Ziarat), and B. rossica O. Gorbunov sp. n. (type locality: Rossia, Astrakhan Region, Dosang).
2) The B. syzcjovi species group with the following two species: B. syzcjovi Gorbunov 1991 (type locality: Georgia: Meskhetian Mts.), B. kappadocica Špatenka 1997, stat. n. (type locality: Turkey: Cappadocia, Yeşilöz).
3) The B. ceiformis species group with a single species: B. ceiformis (Staudinger 1881) (type locality: Kazakhstan: Lepsi).
Bembecia rossica sp. n. is the closest to B. deserticola from the B. dispar taxonomic species group. The male of the new species can be distinguished from that of B. deserticola by the colouration of the abdomen (tergite 2 dorsally black with a few yellow scales distally; tergites 3 and 5 each black with a narrow yellow stripe medially; tergites 4, 6 and 7 each yellow with a row of pale yellow scales with golden sheen distally; yellow ventrally; sternite 1+2 black with a few yellow scales distally in B. deserticola, vs. dorsally tergite 2 black proximally and yellow medially and with a row of pale yellow scales with golden sheen distally; tergites 3 and 5 each narrowly black both proximally and distally and yellow medially; tergites 4 and 6 each yellow with a row of pale yellow scales with golden sheen distally; tergite 7 yellow with a row of black with strong violet-blue sheen scales distally; ventrally sternite 1+2 black proximally and yellow distally in B. rossica sp. n.; compare Figs 3a–3h with Figs 6a–6d), by more numerous orange or yellow-orange scales on the discal spot of the forewing, especially ventrally (dorsally with a few yellow-orange scales distally and ventrally with a small triangular yellow-orange spot distally in the species compared, vs. dorsally the discal spot with orange both proximal and distal margins, ventrally with yellow-orange distal third in B. rossica sp. n.), by the relatively broader discal spot of the forewing (level to vein M2 about one forth broader than external transparent area in B. deserticola, vs. level to vein M2 about one third as broad as external transparent area in B. rossica sp. n.; compare Figs 3a–3h with Figs. 6a–6d), and by the colouration of the outer margin of the hind wing (narrow, about one third as broad as cilia, brown with bronze sheen and a few yellow scales proximally between veins M1 and CuP in B. deserticola, vs. narrow, about 0.5 times as broad as cilia, between veins M1 and CuP dark brown to black with bronze sheen distally and yellow with golden sheen proximally B. rossica sp. n.).
The female of B. rossica sp. n. is separable from the extremely rare orange form of the female of B. deserticola (Figs 6g, 6h) by the largest size (alar expance 17.0 mm in the species compared, vs. 26.2–28.0 mm in B. rossica sp. n.), the colouration of the abdomen (black dorsally; tergites 2, 4 and 6 each with a broad orange stripe distally; tergite 5 with a narrow orange stripe medially; ventrally sternites 1+2 and 3 each black; tergites 4–6 each orange in B. deserticola, vs. dorsally tergite 1 entirely yellow-orange to orange; tergites 2, 4–6 each yellow-orange to orange and a narrow black stripe proximally; tergite 3 yellow-orange to orange distally and black proximally; ventrally orange with an admixture of black scales proximally on sternite 1+2 and 3, and proximal-laterally on sternites 4–6 in B. rossica sp. n.; compare Figs 4a–4h with Figs 6g, 6h) and the forewing ventrally (surface between veins R1–R3 dark brown with bronze sheen in B. deserticola, vs. orange with golden sheen in B. rossica sp. n.), by more developed transparent areas of the forewing (posterior transparent area undeveloped; external transparent area small, divided into four cells between veins R3–M3, level to vein M2 about as broad as discal spot and about 1.6 times broader than apical area in the species compared, vs. about 2.3 times as broad as discal spot and about 3.5 times broader than apical area in B. rossica sp. n.) and less number of orange scales on the surface between veins CuA2 and 1A of the hindwing (surface between veins CuA2 and 1A completely covered with orange scales in B. deserticola; compare Figs 4a–4h with Figs 6g, 6h). From black female of B. deserticola (Figs 6e, 6f), the female of B. rossica sp. n. easily differs by the orange colouration of different parts of the body and wings (compare Figs 4a–4h with Figs 6e, 6f).
From the male of B. tsvetajevi, that of B. rossica sp. n. difers by the colouration of the fore tibia (dorsally black with dark greenish sheen in B. tsvetajevi, vs. dorsally yellow with golden sheen and black base in B. rossica sp. n.), the abdomen (dorsally black with dark greenish sheen; tergite 2 with a narrow yellow stripe distally; tergites 4–6 each with a broad yellow stripe distally; tergite 7 entirely yellow in B. tsvetajevi, vs. dorsally tergite 2 black with greenish-blue sheen proximally and yellow medially; tergites 3 and 5 each narrowly black both proximally and distally and yellow medially; ventrally sternite 1+2 black proximally and yellow distally in B. rossica sp. n.; compare Figs 3a–3h with Figs 1a, 1b) and forewing ventrally (surface between veins R1–R3 dark brown with bronze sheen in the species compared, vs. orange with golden sheen in B. rossica sp. n.). From the yellow form of the female of B. tsvetajevi, the female of B. rossica sp. n. is clearly distinguished by the orange colouration of spaces on different parts of the body and wings (yellow in the species compared), by the well-developed transparent areas of the forewing (forewing completely opaque in B. tsvetajevi), and by the transparent hindwing (completely opaque in the species compared; compare Figs 4a–4h with Figs 1c, 1d).
From the male of B. dispar, the male of B. rossica sp. n. is separable by the colouration of the abdomen (segments 2–7 each with a broad pale yellow to yellow ring distally in the species compared, vs. dorsally tergite 2 black with greenish-blue sheen proximally and yellow medially; tergites 3 and 5 each narrowly black both proximally and distally and yellow medially in B. rossica sp. n.; compare Figs 3a–3h with fig. 232 in Špatenka et al., 1999). The colouration of the female of B. dispar is black, what is a good distinctive sign.
From B. pashtuna, this new species easily differs by the black colouration of both the male and female of B. pashtuna (compare Figs 3 and 4 with figs 1 and 2 in Špatenka, 1997).
The male genitalia of the B. dispar species group are practically indistinguishable. They differ from each other in minute details of crista sacculi only (compare Figs 5a–5e with figs 144, 146–148 in Špatenka et al., 1999). The female genitalia of this species group have also not so distinct differences from each other. They can be separated by relative size of the 8th tergite, antrum and apophysis anterioris (see Fig. 5f and figs 393, 395, 396 in Špatenka et al., 1999).
From other species of the B. dispar ecological species group (B. syzcjovi and B. ceiformis species groups), B. rossica sp. n. clearly differs by the coloration of the abdomen and wings (compare Figs 3 and 4 with Figs 2 and 7). Besides this, the new species can be distinguished from species of these species groups by the conformation of the male genitalia, especially by the shape of the crista sacculi in B. syzcjovi and B. kappadocica (compare Fig. 5b with figs 1 and 2 in Gorbunov, 1989) and crista gnathi in B. ceiformis (compare Fig. 5a with fig. 130 in Špatenka et al., 1999).
Fig. 7.
Bembecia ceiformis (Staudinger 1881). a – male, upside. Kazakhstan, Tarbagatai, Keskesken, 27.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0337–2013. Alar expanse xx.9 mm, b – ditto, underside. Sesiidae Picture № 0338–2013; c – female, upside. Kazakhstan, Tarbagatai, Keskesken, 27.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0339–2013. Alar expanse xx.9 mm, d – ditto, underside. Sesiidae Picture № 0340–2013; e – female, upside. Kazakhstan, Tarbagatai, Keskesken, 27.06.2001, ex l., O. Gorbunov leg. (COGM). Sesiidae picture № 0341–2013. Alar expanse xx.9 mm, f – ditto, underside. Sesiidae Picture № 0342–2013.

Fig. 8.
Bionomics and habitat of Bembecia rossica sp. n. a – Astragalus vulpinus Willd. (Fabaceae), the host plant. Russia, Astrakhan Region, 5 km N of Dosang, 13.05.1997. b – habitat. Russia, Astrakhan Region, 5 km N of Dosang, 13.05.1997. c – freshly emerged male, paratype. Russia, Astrakhan Region, 5 km N of Dosang, 20.08.2013, ex. p., O. Gorbunov leg. Moth emerged 26.08.2013. d – freshly emerged female, paratype. Russia, Astrakhan Region, 5 km N of Dosang, 20.08.2013, ex. p., O. Gorbunov leg. Moth emerged 24.08.2013.
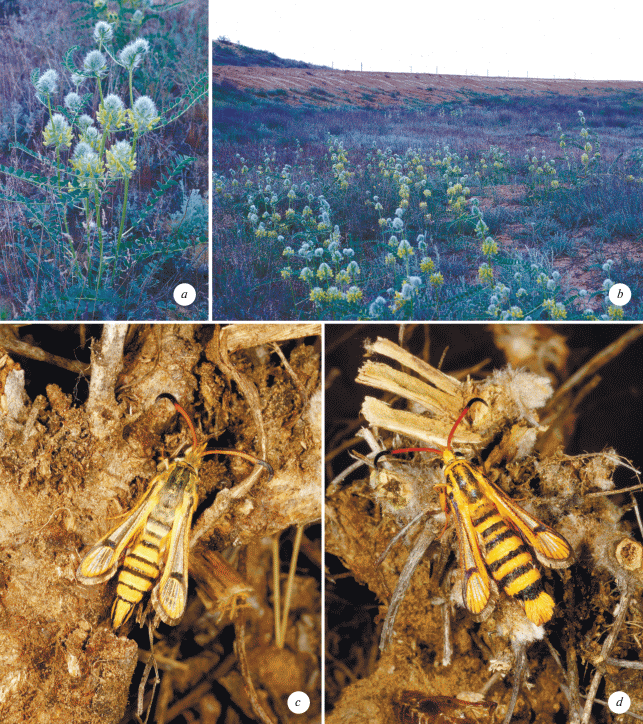
From other congeners, B. rossica sp. n. differs clearly by both the colouration of the different parts of the body and the conformation of both the male genitalia and female genitalia.
Bionomics. The larval host plant is Astragalus vulpinus Willd. (Fabaceae) (Fig. 8a). The larva lives one year inside the root, approximately 3–10 cm below the ground level. Several larvae can inhabit a single root. It depends of the size of the plant. After hibernation, the larva actively feeds in the root. In the beginning of summer it stops eating and tightly closes the food gallery with root fibers, being inside a peculiar “cocoon”. In that “cocoon” the larva have an aestivation till mid August or even mid of September. After the aestivation the larva construcs an exit tube from the root to the ground or, sometimes, to the lower part of the stem of the host plant. In the lower part of the exit tube the larva weaves a dense cocoon with a rounded stopper that opens only from the inside. Adults (Figs 8c, 8d) are on the wing from the end of August to the mid of September.
Habitat. Small depressions among the artemisian semidesert (Fig. 8b) of the Caspian Depression.
Distribution. The new species is known only from the type locality. It is very likely that this new species is widely distributed not only in the Caspian Depression, but also throughout the range of the host plant.
Etymology. This new species is named after Russia, where it inhabits.
Список литературы
Căpuşe I., 1973. Zur Systematik und Morphologie der Typen der Sesiidae (Lepidoptera) in der R. Püngeler-Sammlung des Zoologischen Museums zu Berlin // Mitteilungen der Münchner Entomologischen Gesellschaft. V. 63 P. 134–171.
Efetov K.A., Gorbunov O.G., Ruchko P.V., 2012. Bembecia uroceriformis (Treitschke, 1834) (Lepidoptera: Sesiidae) in Ukraine // Tavricheskiy Mediko-biologicheskiy Vestnik. V. 15. № 2. P. 336–337. [in Russian]
Gorbunov O.G., 1989. A new species of the genus Bembecia Hübner, 1819 from the Caucasus, USSR (Lep., Sesiidae) // Atalanta. V. 20. P. 119–123.
Gorbunov O.G., 2008. Sesiidae // Sinev S.Yu. (Ed.), Catalogue of the Lepidoptera of Russia. M., Sankt Petersburg: KMK Scientific Press. P. 110–112. [in Russian]
Gorbunov O.G., 2018. New data on the clearwing moth fauna of the Altai Mountains, Russia, with the description of two new species (Lepidoptera, Sesiidae) // Zootaxa. V. 4425. № 2. P. 263–282.
Gorbunov O.G., Efetov K.A., 2018. The clearwing moth genus Bembecia Hübner 1819 [“1816”] (Lepidoptera, Sesiidae) in Crimea, with the description of a new species // Zoologicheskiy zhurnal. V. 97. № 7. P. 812–839.
Gorbunov O.G., Krupitsky A.V., Marusov A.A., 2017. A new species of Bembecia from China, with a catalogue of Chinese species of the genus (Lepidoptera: Sesiidae) // Zootaxa. V. 4273. № 4. P. 559–575.
Kallies A., Bartsch D., 2010. Revision of some types of the genus Bembecia Hübner, 1819 in the Püngeler collection in the Museum for Natural History of the Humboldt University, Berlin (Sesiidae) // Nota lepidopterologica. V. 33. № 1. P. 81–84.
Püngeler R., 1912. D.[ipsosphecia] polysona Püng. // 25. Family: Aegeriidae (Sesiidae) // Bartel M. // Seitz A. (Ed.), 1907–1913, The Macrolepidoptera of the World. I. Division: the Macrolepidoptera of the Palearctic Fauna. V. 2. The Palearctic Bombyces & Sphinges. Stuttgart: Verlag des Seitz’schen Werkes (A. Kernen). P. 375–416. Pls 50–52.
Špatenka K., 1997. Neue Glasflügler (Sesiidae, Lepidoptera) aus dem Pamir und dem Hindukusch // Bonner zoologische Beiträge. V. 47. № 1–2. P. 31–41.
Špatenka K., 1997a. Neue Glasflügler-Arten und Unterarten aus Europa und der Türkei (Sesiidae, Lepidoptera) // Bonner zoologische Beiträge. V. 47. № 1–2. P. 43–57.
Špatenka K., Gorbunov O., Laštůvka Z., Toševski I., Arita Y., 1999. Sesiidae, Clearwing Moths // Naumann C.M. (Ed.), Handbook of Palaearctic Macrolepidoptera. V. 1. Wallingford: Gem Publishing Company. 569 p.
Špatenka K., Petersen M., Kallies A., 1997. Ergebnisse einer sesiidologischen Expedition 1994 nach Kasachstan und Kirgistan (Lepidoptera: Sesiidae) // Nachrichten des entomologischen Vereins Apollo, N.F. V. 17. № 4. P. 405–422.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал


