Зоологический журнал, 2023, T. 102, № 2, стр. 153-162
Contribution to the knowledge of the oribatid mite genus Aleurodamaeus (Acari, Oribatida, Aleurodamaeidae), with description of a new species from Ethiopia
S. G. Ermilov a, *, E. A. Hugo-Coetzee b, **, L. B. Rybalov c, ***
a Tyumen State University
625003 Tyumen, Russia
b National Museum, Bloemfontein, 9301 South Africa; University of the Free State,
9301 Bloemfontein, South Africa
c Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: ermilovacari@yandex.ru
** E-mail: lhugo@nasmus.co.za
*** E-mail: lrybalov52@mail.ru
Поступила в редакцию 19.05.2022
После доработки 24.05.2022
Принята к публикации 26.05.2022
- EDN: HQSYXO
- DOI: 10.31857/S0044513423020058
Аннотация
A new species of the genus Aleurodamaeus (Oribatida, Aleurodamaeidae) is described from heather forest litter in central Ethiopia. Aleurodamaeus aethiopicus sp. n. differs from all related species of the genus by the presence of a thick cerotegument with specific ornamentations (densely cellular with a median longitudinal interruption) on the notogaster. An identification key to all known species of Aleurodamaeus is provided.
The oribatid mite genus Aleurodamaeus (Acari, Oribatida, Aleurodamaeidae) was proposed by Grandjean (1954) with Damaeus setosus Berlese 1883 as type species. So far, it comprises 17 species, which are collectively distributed in the Afrotropical, Neotropical and southern Palaearctic regions (Subías, 2004, online version 2022). The revised generic diagnosis and an identification key to nine species of Aleurodamaeus from South Africa were provided by Hugo-Coetzee (2013). Grandjean (1954) characterized Aleurodamaeus as retaining the exuvial scalps in the adult stage, while Hugo-Coetzee (2013) stated that the exuvial scalps are retained as adults, but they are weakly attached and are easily lost so that only a few or not any individuals with scalps may be found in a sample. However, there are clearly species that either retain the scalps or do not retain the scalps, i.e. there are a complete absence of scalps in all the studied individuals and also no loose scalps in the sample. Therefore, in our opinion, Aleurodamaeus adults either retain the scalps or not. Unfortunately ontogenetic studies of Aleurodamaeus are scare with only juveniles of A. africanus and A. setosus known (both species retain the exuviae in the adult stage) (Norton, Ermilov, 2014). Ontogenetic studies (of preferably juveniles bred in the laboratory) of species which do not retain the exuviae in the adult phase are necessary to clarify this characteristic.
The main goal of our paper is to describe and illustrate a new species of Aleurodamaeus, based on adults, collected from Ethiopia. Presently, the Ethiopian mite fauna is insufficiently studied, and only two species of the genus have been registered (e.g. Ermilov et al., 2010; Ermilov, Rybalov, 2012): A. africanus Mahunka 1984; A. recenfesevpi Ermilov et Rybalov 2012. The additional goal of our paper is to present an identification key to all known species of Aleurodamaeus.
METHODS
Observation and documentation. Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in dorsal view, from the tip of the rostrum to the posterior edge of the notogaster. Notogastral width refers to the maximum width of the notogaster in dorsal view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu-tibia-tarsus. Drawings were made with a camera lucida using a Leica transmission light microscope “Leica DM 2500”. For SEM microscopy alcohol preserved mites were dusted with gold and scanned with the aid of a TESCAN Mira3 LMU SEM microscope.
Terminology and conventions. General morphological terminology used in this paper mostly follows that of Grandjean (see Travé and Vachon (1975) for references), Norton (1977), and Norton and Behan-Pelletier (2009).
Abbreviations. Prodorsum: ro, le, in, bs, ex = = rostral, lamellar, interlamellar, bothridial, and exobothridial seta, respectively. Notogaster: h, p = notogastral setae; ia, im, ip, ih, ips = notogastral lyrifissures; gla = opisthonotal gland opening. Gnathosoma: a, m, h = subcapitular setae; or = adoral seta; d, l, sup, inf, cm, ul, sul, vt, lt = palp setae; ω = palp solenidion; cha, chb = cheliceral setae; Tg = Trägårdh’s organ. Epimeral and lateral podosomal regions: 1a–c, 2a, 3a–c, 4a–c = epimeral setae; PdI, PdII = pedotectum I, II, respectively. Anogenital region: g, ag, an, ad = genital, aggenital, anal, and adanal seta, respectively; iad = adanal lyrifissure; p.o. = preanal organ. Legs: Tr, Fe, Ge, Ti, Ta = trochanter, femur, genu, tibia, and tarsus, respectively; ω, φ, σ = solenidia; ɛ = famulus; d, l, v, bv, ev, ft, tc, it, p, u, a, s, pv, pl = leg setae; p.a. = porose area.
TAXONOMY
Aleurodamaeus aethiopicus Ermilov, Hugo-Coetzee et Rybalov sp. n. (Figs 1–6)
Fig. 1.
Aleurodamaeus aethiopicus sp. n., adult: a – dorsal view (not shown: legs); b – dorsal view (not shown: legs, prodorsal and notogastral cerotegument); c – right lateral view (not shown: gnathosoma, legs, prodorsal and notogastral cerotegument). Scale bar 100 μm.
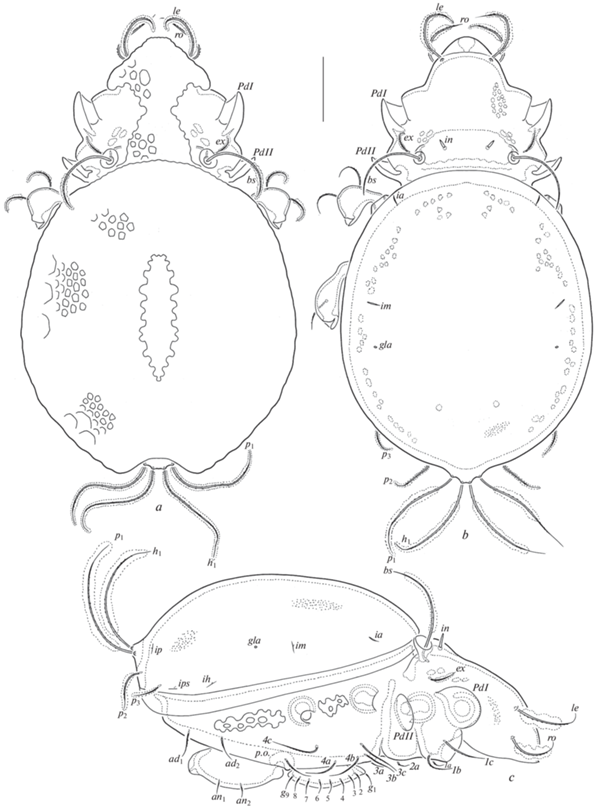
Fig. 2.
Aleurodamaeus aethiopicus sp. n., adult: a – ventral view (not shown: gnathosoma, legs); b – posterior view; c – subcapitulum, ventral view; d – palp, left, paraxial view; e – chelicera, left, paraxial view. Scale bar (μm): a, b – 100; c, e – 50; d – 20.
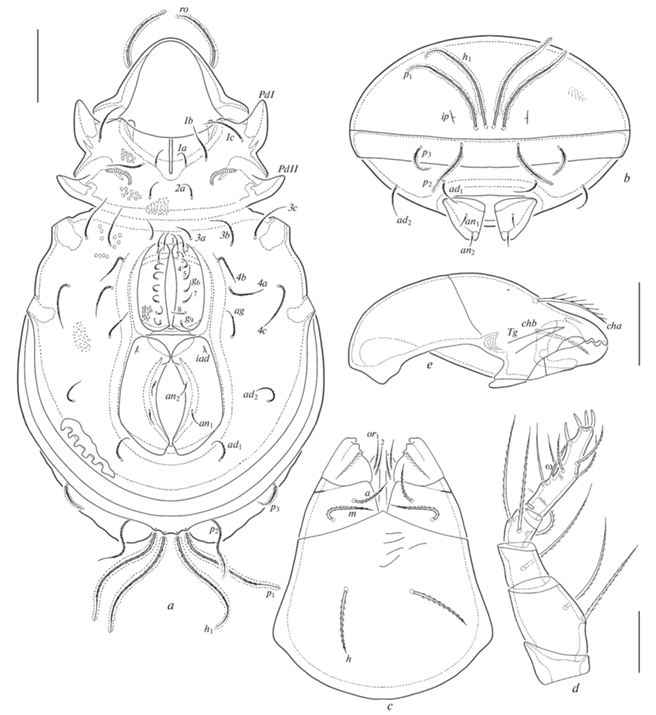
Fig. 3.
Aleurodamaeus aethiopicus sp. n., adult: a – leg I, right, antiaxial view; b – leg II, without tarsus, right, antiaxial view; c – leg III, without tarsus, right, ventral view; d – leg IV, left, antiaxial view. Scale bar 50 μm.
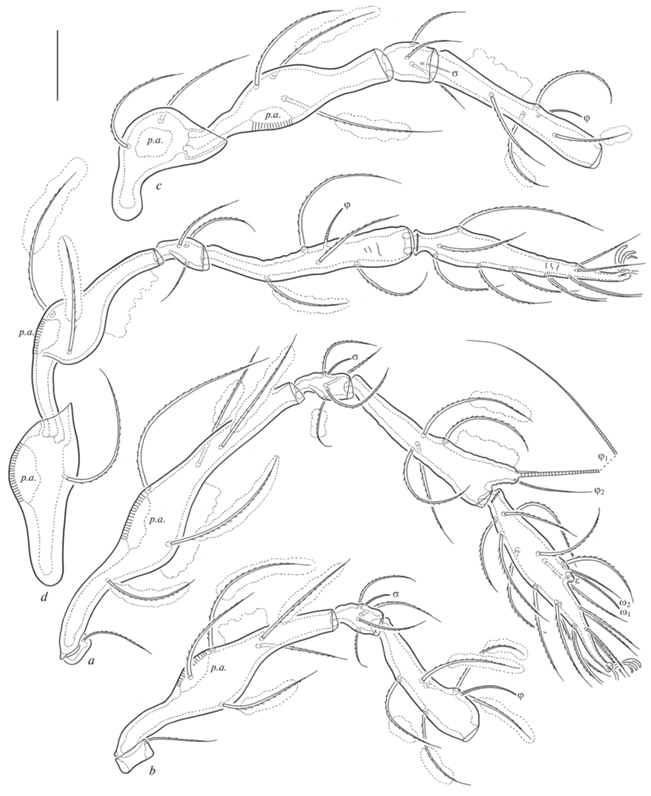
Fig. 4.
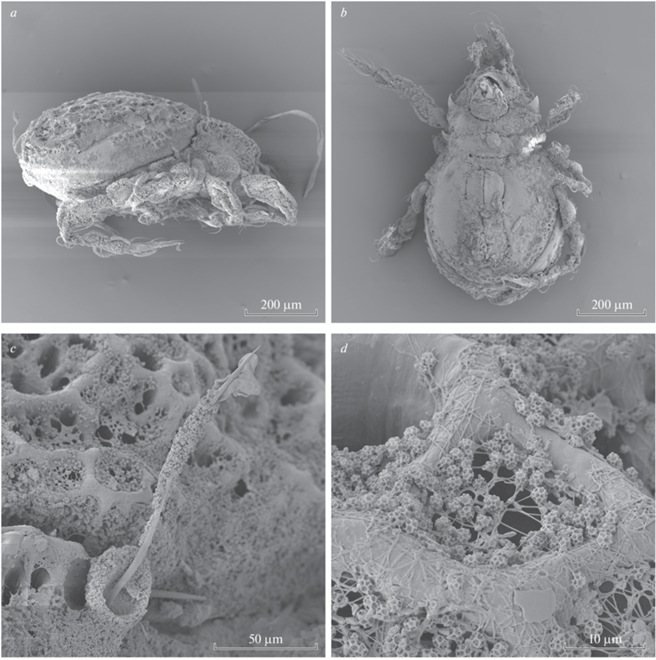
Aleurodamaeus aethiopicus sp. n., adult, SEM micrographs: a, b – dorsal view; c – anterodorsal view; d – posterior view.
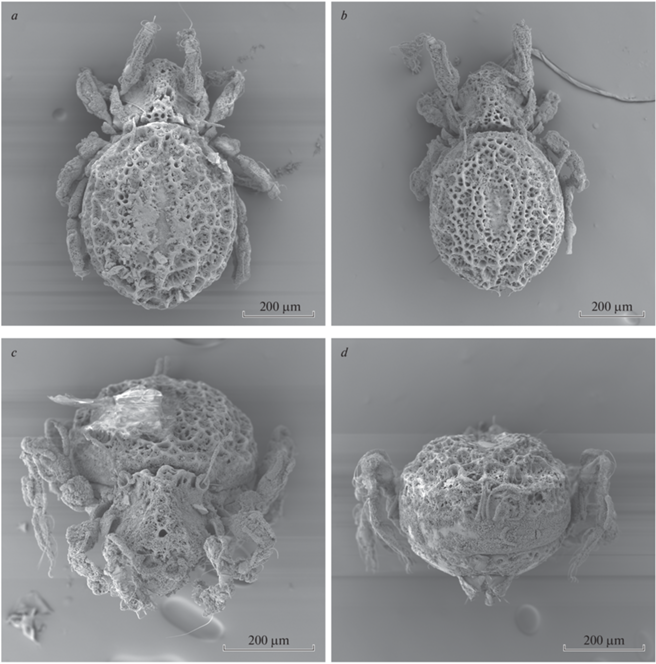
Fig. 5.
Aleurodamaeus aethiopicus sp. n., adult, SEM micrographs: a – right lateral view; b – ventral view; c – bothridium and bothridial seta; d – notogastral cerotegument.
Material. Holotype and 20 paratypes: Ethiopia, Oromia Region, Arsi Zone, Arsi Mountains National Park, Mount Chilalo, 07°56′09.5″ N, 039°11′54.7″ E, 3177 m a.s.l., point № 8, sifting litter under Hypericum sp., Thymus sp. and green mosses in heather bushes (Erica arborea), Berlese’s funnels, 27.11.2021 (leg. L.B. Rybalov) (Fig. 6).
The holotype is deposited in the collection of the Senckenberg Museum of Natural History, Görlitz, Germany; 20 paratypes are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia. All specimens are preserved in 70% solution of ethanol with a drop of glycerol.
Diagnosis. Body length: 713–830. Notogaster with thick, gel-like, densely cellular cerotegument, with a longitudinally elongate central part with interrupted or very thin cerotegument. Rostral and lamellar setae setiform, barbed; interlamellar seta spiniform, roughened; bothridial seta long, rod-like, roughened. Exuvial scalps always absent. Four pairs of notogastral setae barbed (h1, p1 very long, flagellate; p2 long, flagellate; p3 medium-sized, setiform). Epimeral and anogenital setae setiform, barbed. Nine pairs of genital setae. Two pairs of adanal setae. Discidium and parastigmatic enantiophysis absent. Leg famulus sunken in cylindrical apophysis.
Description. Measurements. Large species. Body length: 796 (holotype), 713–830 (20 paratypes); body width: 498 (holotype), 448–514 (20 paratypes). Sex not identified.
Integument. Body color brown. Surface with dense microtuberculate sculpturing (visible under high magnification in dissected specimens). Notogaster, dorsal part of prodorsum and marginal zone of ventral plate covered by thick (large masses), gel-like, densely cellular layer of cerotegument; central part of notogastral with longitudinally elongated region with interrupted or very thin cerotegument. Body, legs and setae partially covered by dense microgranules and filaments; microgranule is hollow and multicellular (Fig. 5c, 5d).
Prodorsum. Rostrum broadly rounded. Rostral (123–143), lamellar (143–154) and exobothridial (53–61) setae setiform, flexible, slightly barbed. Interlamellar seta (24–32) spiniform, roughened. Bothridial seta (164–172) rod-like, roughened.
Notogaster. Exuvial scalps always absent. Dorsosejugal furrow deep, narrow. Four pairs of flexible, slightly barbed setae; h1, p1 (205–287) and p2 (90–98) flagellate, p3 (53–61) setiform. Opisthonotal gland opening and all lyrifissures distinct.
Gnathosoma. Subcapitulum size: 164–184 × 123–143. Subcapitular setae (a: 32–41; m, h: 45–49) and adoral (20–24) setae setiform, flexible, slightly barbed. Palp (length: 94–102) setation: 0-2-1-3-9(+ω). Postpalpal seta (6) spiniform, smooth. Chelicera (length: 164–184) with two setiform, barbed setae (cha: 45–53; chb: 36–41).
Epimeral and lateral podosomal regions. Epimeral setal formula: 3-1-3-3; setae (1a, 2a, 3a: 32–41; 1b, 3b, 4a, 4b: 53–61; 1c, 3c, 4c: 86–98) setiform, flexible, slightly barbed. Discidium and tubercles of parastigmatic enantiophysis S absent.
Anogenital region. Genital (32–41), aggenital (49–61), anal (24–32), and adanal (49–61) setae setiform, flexible, slightly barbed. Nine pairs of genital setae and two pairs of adanal setae present. Adanal lyrifissure poorly observed.
Legs. Tridactylous; median claw slightly thicker than lateral claws, all roughened on dorsal side. Porose area on leg femora I-IV and trochanters III, IV well observed. Formulas of leg setation and solenidia: I (1-5-4-5-20) [1-2-2], II (1-5-4-5-16) [1-1-2], III (2-3-3-4-15) [1-1-0], IV (1-2-3-4-13) [0-1-0]; homology of setae and solenidia indicated in Table 1. Famulus on tarsus I minute, sunken in cylindrical apophysis. Seta s on tarsus I setiform, barbed (not eupathidial), located between setae a and pv.
Table 1.
Leg setation and solenidia of adult Aleurodamaeus aethiopicus sp. n.
| Leg | Tr | Fe | Ge | Ti | Ta |
|---|---|---|---|---|---|
| I | v' | d, (l), bv'', v'' | d, (l), v', σ | (l), (v), dφ1, φ2 | (ft), (tc), (it), (p), (u), (a), s, (pv), l '', v', (pl), ɛ, ω1, ω2 |
| II | v' | d, (l), bv'', v'' | d, (l), v', σ | d, (l), (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv), l '', ω1, ω2 |
| III | l ', v' | d, l ', ev' | d, l ', v', σ | d, l ', (v), φ | (ft), (tc), (it), (p), (u), (a), s, (pv) |
| IV | v' | d, ev' | d, l ', v' | d, l ', (v), φ | (ft), (tc), (p), (u), (a), s, (pv) |
Remarks. In having four pairs of notogastral setae (e.g. h1, p1 very long, flagellate; p2 long, flagellate; p3 medium-sized, setiform), thick and gel-like cerotegument on the notogaster, two pairs of adanal setae, rod-like bothridial seta, and sunken leg famulus, Aleurodamaeus aethiopicus sp. n. is most similar to A. woasi Hugo-Coetzee, 2013 from South Africa. However, the new species can be distinguished from the latter by the larger body length (713–830 versus 318–420), the presence of densely cellular cerotegumental layer on the notogaster (versus cerotegument represented by large polygonal parts), the number of genital setae (nine pairs versus seven pairs), and the absence (versus presence) of parastigmatic tubercle Sp.
Etymology. The species name aethiopicus refers to the country of origin, Ethiopia.
KEY TO KNOWN SPECIES OF ALEURODAMAEUS
Aleurodamaeus hungaricus Paschoal et Johnson 1985 from south-central Europe and A. trichosus (Kulijev 1979) from Caucasus are excluded from this key. The description of A. hungaricus is complicated and confusing, with much information on the ratio of leg segments, but little about the characteristics of the body. It has a body length of 504, cerotegument piled on prodorsum and notogaster, long bothridial seta, four pairs of notogastral setae, all on tubercles, with h1, p2 (could be p1) long, seven pairs of genital setae, two pairs of adanal setae with ad1 posterolateral to anal plates and ad2 paraanal (Paschoal, Johnson, 1985). Aleurodamaeus trichosus is also not well described. It has a body length of 775, presumably three pairs of notogastral setae, all subequal in length, long flagellate bothridial seta, cerotegumental granules on the notogaster and cerotegument in a sideways ‘l’ form on both sides on the prodorsum (Kulijev, 1979).
1 Five pairs of notogastral setae, i.e. seta h2 present …………………………………………......................... 2
– Three11 or four pairs of notogastral setae, i.e. seta h2 absent ………………………………............................ 3
2 Notogastral seta h2 setiform, curving laterally; anterior transverse ridge on notogaster absent; cerotegument on prodorsum in the form of a sideways ‘m’ on both sides; body length: 491–555 .….. A. salvadordalii Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: South Africa.
– Notogastral seta h2 flexible, twisted; anterior transverse ridge on notogaster present; cerotegument on prodorsum in the form of an ‘A’; body length: 455–550 …………………................A. deswardti (Hugo 2010) (see Hugo, 2010). Distribution: South Africa.
3 All notogastral setae distinctly shorter than the bothridial seta ………………………….......................... 4
– All or some notogastral setae similar or longer in length to the bothridial seta …………......................... 7
4 Large masses of notogastral cerotegument forming four longitudinal bands; prodorsum with a large mass of cerotegument; body length: 359–439 ………. A. angelae Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: South Africa.
– Large masses of notogastral cerotegument mostly absent, except sometimes anterior and posterior parts of notogaster; prodorsum without a large mass of cerotegument or with narrow cerotegument across the lamellar region …………………………………………….... 5
5 Large masses of cerotegument present in anterior and posterior parts of the notogaster; insertions of notogastral setae p2 and p3 very close to each other, almost touching; body length: 491–550 …... A. vicinus Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: Afrotropical region.
– Large masses of cerotegument absent in anterior and posterior parts of the notogaster; insertions of notogastral setae p2 and p3 clearly distanced from each other …………………................................................. 6
6 Notogastral setae h1 and p1 much longer than p2 and p3; all notogastral setae smooth; body length: 319–367 …………………………………………………A. minutus Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: South Africa.
– Notogastral setae h1, p1, p2, and p3 similar in length; all notogastral setae ciliate; body length: 431–481 ……………………………………A. murombodziensis Ermilov 2021 (see Ermilov, Bąkowski, 2021). Distribution: Mozambique.
7 Exuvial scalps present on the notogaster ………. 8
– Exuvial scalps absent on the notogaster ……… 10
8 Nine to ten pairs of genital setae; body length: 662–739 …………………………………….......A. africanus Mahunka 1984 (see Mahunka, 1984). Distribution: Afrotropical region.
– Seven pairs of genital setae ………………………… 9
9 Notogastral setae h1 and p1 long, similar in length, both inserted on strong tubercles; body length: 550–660 …………………………………………………A. setosus (Berlese 1883) (see Berlese, 1883; Pérez-Íñigo, 1970, 1986; Seniczak et al., 2012). Distribution: southern Palaearctic region, Mexico.
– Notogastral seta h1 long, distinctly longer than medium-sized seta p1, both not inserted on tubercles; body length: 727–858 ………………………A. ermilovi (Hugo-Coetzee 2014) (see Hugo-Coetzee, 2014). Distribution: South Africa.
10 Discidium well developed, prominent ………. 11
– Discidium weak or absent ………………………… 12
11 Large masses of cerotegument in central part of notogaster forming two longitudinal parallel bands; two pairs of adanal setae; body length: 543–620 …….........................................…A. prominens Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: South Africa.
– Large masses of cerotegument in central part of notogaster forming elongate diamond-shaped structure; three pairs of adanal setae; body length: 770 …….........................................A. cephalotes (Berlese 1916) (see Berlese, 1916; Mahunka, Mahunka-Papp, 1995). Distribution: Afrotropical region.
12 Large masses of cerotegument in central part of notogaster forming three longitudinal parallel bands ………………………………………………………….. 13
– Large masses of cerotegument in central part of notogaster not forming longitudinal parallel bands …………………………………………………………. 14
13 Notogastral setae p1 and h1 similar in length; adanal setae ad1 located posterior to anal aperture; body length: 547–664 …………………………A. recenfesevpi Ermilov et Rybalov 2012 (see Ermilov, Rybalov, 2012). Distribution: Ethiopia.
– Notogastral seta p1 longer than h1; adanal setae ad1 located lateral to anal aperture; body length: 447–532 ……………………………………………… A. niedbalai Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: South Africa.
14 Nine pairs of genital setae; body length: 713–830 ……………………………………… A. aethiopicus sp. n. Distribution: Ethiopia.
– Seven pairs of genital setae ………………………. 15
15 Large masses of notogastral cerotegument forming polygonal structures; cerotegument on prodorsum without alveoli; anterior transverse ridge on notogaster present; body length: 318–420 …………………………
……….. A. woasi Hugo-Coetzee 2013 (see Hugo-Coetzee, 2013). Distribution: South Africa.
– Large masses of notogastral cerotegument with craterlike alveoli and lines; cerotegument on prodorsum with small alveoli; anterior transverse ridge on notogaster absent; body length: 430 ………………………
……….. A. australis Woas 1992 (see Woas, 1992). Distribution: South Africa, Mozambique.
Список литературы
Berlese A., 1883. Sopra due nuovi generi di Acari italiani // Lettura fatta alla R. Accademia di Padova. Atti R. Accademia, Padova. Vol. 33. P. 45–52.
Berlese A., 1916. Centuria prima di Acari nuovi // Redia. Vol. 12. P. 19–67.
Ermilov S.G., Bąkowski M., 2021. Three new species of oribatid mites (Acari, Oribatida) from Mozambique // Systematic and Applied Acarology. Vol. 26. № 5. P. 885–901.
Ermilov S.G., Rybalov L.B., 2012. A new species of Aleurodamaeus from Ethiopia, with remarks on the taxonomic status of Aleurodamaeus (Trichodamaeus) Mahunka, 1984 (Acari: Oribatida: Aleurodamaeidae) // Opuscula Zoologica Budapest. Vol. 43. № 1. P. 21–26.
Ermilov S.G., Sidorchuk E.A., Rybalov L.B., 2010. Morphology of juvenile stages of Pedrocortesella africana Pletzen, 1963 and Aleurodamaeus africanus Mahunka, 1984 (Acari, Oribatida) // Annales Zoologici. Vol. 60. № 3. P. 391–406.
Grandjean F., 1954. Observations sur les Oribates (28e serie) I – sur les Gymnodamaeidae // Bulletin de Muséum national d’Histoire naturelle. Vol. 26. № 2. P. 204–211.
Hugo E.A., 2010. Two new species of Gymnodamaeidae (Acari: Oribatida) from South Africa // International Journal of Acarology. Vol. 36. № 3. P. 199–210.
Hugo-Coetzee E.A., 2013. New species of Aleurodamaeus Grandjean, 1954 (Oribatida: Aleurodamaeidae) from South Africa // Zootaxa. Vol. 367. № 4. P. 531–556.
Hugo-Coetzee E.A., 2014. A new species of Adrodamaeus (Acari, Oribatida, Gymnodamaeidae) from South Africa // Navorsinge van die Nasionale Museum, Bloemfontein. Vol. 30. № 6. P. 87–99.
Kulijev K.A., 1979. New species of oribatid mites of the genera Plesiodamaeus, Allodamaeus, Carabodes, Hermanniella // Doklady Academii Nauk Azerbaijan SSR. Vol. 35. № 12. P. 78–83.
Mahunka S., 1984. Oribatids of the Eastern Part of the Ethiopian Region (Acari). V // Acta Zoologica Hungarica. Vol. 30. № 1–2. P. 87–136.
Mahunka S., Mahunka-Papp L., 1995. The oribatid species described by Berlese (Acari). Budapest: Hungarian Natural History Museum. 325 p.
Norton R.A., 1977. A review of F. Grandjean’s system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae // Dindal D.L., ed. Biology of oribatid mites. Syracuse, SUNY College of Environmental Science and Forestry. P. 33–61.
Norton R.A., Behan-Pelletier V.M., 2009. Oribatida // A Manual of Acarology (TX). Lubbock: Texas Tech University Press. P. 430–564.
Norton R.A., Ermilov S.G., 2014. Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida) // Zootaxa. Vol. 3833. № 1. P. 1–132.
Paschoal A.D., Johnston D.E., 1985. Aleurodamaeidae (Acari: Oribatei) a new family of Oribatid, with a description of Aleurodamaeus hungaricus sp. n. // Revista brasileira de entomologia. Vol. 29. № 1. P. 21–26.
Pérez-Íñigo C., 1970. Acaros Oribatidos de suelos de Espania Peninsular e islas Baleares (Acari, Oribatei) Parte II // Eos. Revista española de entomología Madrid. Vol. 45. P. 241–317.
Pérez-Íñigo C., 1986. Contribucion al conocimiento de los Oribatidos (Acari, Oribatei) de la Gomera (Islas Canarias) // Eos. Revista española de entomología Madrid. Vol. 62. P. 187–208.
Seniczak S., Ayyıldız N., Seniczak, A., 2012. Setal losses in the dorsal hysterosoma of Plateremaeoidea (Acari: Oribatida) in the light of ontogenetic studies // Journal of Natural History. Vol. 46. № 7–8. P. 411–451.
Subías L.S., 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758–2002) // Graellsia. Vol. 60. Número extraordinario. P. 3–305.
Subías L.S., 2022. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). 17a actualización. 537 p. Available from: bba.bioucm.es/cont/docs/ RO_1.pdf.
Travé J., Vachon M., 1975. François Grandjean. 1882–1975 (Notice biographique et bibliographique) // Acarologia. Vol. 17. № 1. P. 1–19.
Woas S., 1992. Beitrag zur Revision der Gymnodamaeidae Grandjean, 1954 (Acari, Oribatei) // Andrias. Vol. 9. P. 121–161.
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал



