Зоологический журнал, 2022, T. 101, № 2, стр. 145-152
Epibiotic Pedunculate Barnacles on the Swimming Crab Monomia Haanii (Decapoda, Portunidae) from the Nha Trang Bay, Vietnam
Le Thi Kieu Oanh a, *, Nguyen Phuong Lien a, Vo Thi Ha a, Nguyen Thi Hai Thanh a, Fedor Lishchenko a, b
a Coastal Branch of the Vietnam–Russia Tropical Center, № 30
Nguyen Thien Thuat rd, Nha Trang, Khanh Hoa, Vietnam
b Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences
119071 Moscow, Russia
* E-mail: filiusferro@gmail.com
Поступила в редакцию 11.12.2020
После доработки 11.03.2021
Принята к публикации 13.03.2021
- EDN: HERWPK
- DOI: 10.31857/S0044513422020052
Аннотация
The crab, Monomia haanii is a portunid species common in Vietnam. However, the symbiotic fauna of this crab is still not well known. The study on the infestation rate of epibiotic pedunculate barnacles on M. haanii was conducted in 2019. Individuals of M. haanii, including 428 males and 266 females, were randomly selected from the catches of fishing boats operating in the Nha Trang Bay. More than 50% crabs were infested by barnacles belonging to four species: Octolasmis angulata, O. neptuni, O. warwicki, and Dianajonesia tridens. Among these, O. angulata was the dominant species with the highest prevalence (49.6%) and a relatively high median intensity (6 barnacle individuals per host) of infestation. The infestation by barnacles did not differ significantly between male and female crabs, nor did it vary between ovigerous and non-ovigerous individuals. A strongly positive correlation between the infestation and host size was found. The infestation was not observed in crabs less than 40 mm in carapace width. Two species, O. angulata and O. neptuni, colonized only host branchial chambers, O. warwicki occupied exclusively external integuments, while D. tridens was found in both gill chambers and at the margin of the crab carapace. In host branchial chambers, barnacles were the most abundant on gills, especially on gill number 4. The crab’s exoskeleton was inhabited only by O. warwicki. This species preferred to settle on the carapace rather than on the appendages.
While life forms of stalked barnacles vary from free-living to sessile, barnacles of genera of Octolasmis Gray 1825 and Dianajonesia Koçak et Kemal 2008 are epibionts on crustaceans, especially decapod species (Jeffries et al., 1982). The life cycle of these barnacles is determined by the ecdysis of their host. The cyprid larvae approach and permanently attach to an appropriate site on the outer surface of the carapace or in the branchial chambers of recently molted decapods. At the attached site, epibiotic larvae experience metamorphic processes into juvenile and adult barnacles. When the host experiences ecdysis, the barnacle’s life cycle ends (Jeffries et al., 1989; Voris et al., 2000; Santos, Bueno, 2002).
Vast majority of epibiotic barnacles do not feed on host’s tissue. They use host integument as substrate, for protection and exploit the host’s movements for accessing the wider resource base and for larval dispersal (Gannon, Wheatly, 1992; Voris et al., 2000). However, massive infestation by these barnacles may lead to a reduction of host movement and respiration, weakening or even killing the host (Gannon, Wheatly, 1992; Hudson, Lester, 1994).
The composition of pedunculate barnacle species fauna, especially octolasmids, dianajonesids and the relationship between them and the decapod host was studied rather intensively (Jeffries et al., 1982; Jeffries et al., 2005; Hassan et al., 2019; Jeffries, Voris, 2005; Kumaravel et al., 2009; Rasheed, Mustaquim, 2017). In the Vietnamese waters, barnacle infestation status and species composition were studied in 2 crab species, Portunus pelagicus and P. sanguinolentus (Oanh et al., 2018, 2018a). The molecular phylogenetics based on the mitochondrial cytochrome oxidase gene subunit I (COI) of pedunculate barnacles infesting these species was analyzed (Binh et al., 2018).
Swimming crab Monomia haanii Stimpson 1858 is a common and commercially exploited species distributed in the Indo-Pacific region (Chertoprud et al., 2012). This species has high-quality meat and is exported to highly regulated markets, such as the American one. The biological characteristics and molecular phylogenetics of this crab were addressed in several studies (Windsor et al., 2019; Chertoprud et al., 2012), however, the symbiotic was not studied yet. This study aims to determine species composition, distribution of epibiotic pedunculate barnacles on M. haanii, and analyze the impact of host sex and size on the prevalence and intensity of infestation by these barnacles.
MATERIALS AND METHODS
Sample collection and treatment
Specimens of Monomia haanii Stimpson 1858 were randomly collected from the fishing boats, operating in Nha Trang Bay from January to December of 2019. The specimens were transported to the laboratory, kept in the fridge at the temperature of 4°C and dissected within two days after collecting. The sex of each crab was determined and its size (carapace width, the distance between the tips of two longest epibranchial spines, ±0.1 mm) was measured. After examining the dorsal and ventral surface of crabs, carapace and abdomen were dissected. The dissecting was performed under the Olympus SZ61 stereomicroscope. Epibiont barnacles were collected and identified on the basis of morphological features as described by Jefferies et al. (2005).
The size of the studied crabs ranged from 36 to 93 mm. For the purpose of examining the infestation state by crab size, the sample was divided into 6 groups by crab’s carapace width (<40 mm, 40–49, 40–59, 60–69, 70–79 mm and ≥80 mm) (Li et al., 2014).
The data collected did not allow us to assess the impact of sampling time (by months) on infestation by barnacles.
The state of infestation by epibiont barnacles was expressed in prevalence and intensity. Prevalence of infestation (%) is the number of hosts infected by epibiont barnacles divided by the number of examined crabs. Intensity of infestation (median (minimum-maximum)) is the number of barnacles in a single infested crab (Bush et al., 1997).
Order in which barnacle species were arranged in the results and discussion sections was determined by location of their settlement – from the branchial chamber to the external surface of the carapace.
Statistical analyses
Statistical Product and Service Solutions (SPSS) software ver. 24.0 was used for statistical analyses.
As the distribution of epibiont barnacles was non-normal distribution, statistical analysis of infection state was performed by utilizing non-parametric tests, the data of median intensity was used instead of mean intensity. Differences in all tests were considered significant at p < 0.05 (Li et al., 2014).
The difference in the prevalence of infestation between male and female crabs was studied with the Chi-square test.
Mann–Whitney test was used to test the difference in median intensity of infestation for males and females, ovigerous and non-ovigerous female crabs.
Non-parametric tests of Kruskal-Wallis was applied to test whether the median intensities were the same among different gills from 1 to 8 of the crabs.
Spearman’s correlation test was used to assess the relationships between infestation by barnacles and host size.
RESULTS
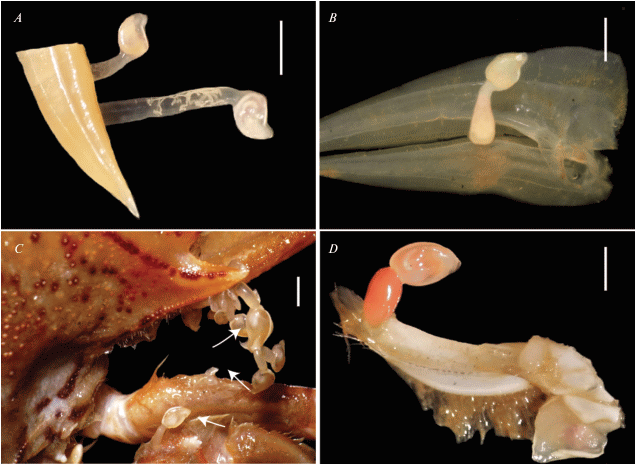
Among 694 individuals of Monomia haanii examined, including 428 males and 266 females, 366 crabs (52.7%) were infested by epibiotic pedunculate barnacles. These barnacles belong to 4 species: Octolasmis angulata, O. neptuni, O. warwicki and Dianajonesia tridens (Fig. 1).
Fig. 1.
The epibiotic pedunculate barnacle infested portunid crab Momomia haanii: A – Octolasmis angulata on gill, B – O. neptuni on gill, C – O. warwicki on carapace, D – Dianajonesia tridens on maxilliped. Scale bars: 3 mm.
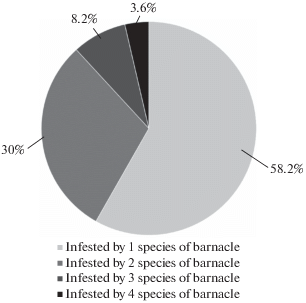
Each host was infested by 1 to 4 epibiont species. There is a clear negative correlation between the number of barnacle species infesting the crabs and the percentage of such crabs among infested crabs. The percentage of crabs hosting only 1 barnacle species was the highest (58.2% of infested crabs), while the percentage of crabs hosting all 4 barnacle species reached only 3.6% (Fig. 2).
The prevalence of infestation by O. angulata on M. haanii was 49.6%, which was markedly higher than this of other barnacles. O. angulata and O. warwicki had the highest level of infestation intensity, 6 (1–97) and 6 (1–186) barnacles per host, respectively (Table 1).
Table 1.
The species composition of epibiotic pedunculate barnacles, prevalence (Prev, %), median and min, max of intensity (Md inten (Min–Max), barnacle individuals per host) of infestation by these barnacles in general and by sexes of Monomia haanii crabs
| Epibiont barnacles | Total crabs (N = 694) | Male crabs (N = 428) | Female crabs (N = 266) | |||
|---|---|---|---|---|---|---|
| Prev, % | Md inten (Min–Max) |
Prev, % | Md inten (Min–Max) |
Prev, % | Md inten (Min–Max) |
|
| O. angulata | 49.6 | 6 (1–97) | 48.4 | 6 (1–97) | 51.5 | 6 (1–92) |
| O. neptuni | 9.9 | 2 (1–36) | 11.5 | 3 (1–36) | 7.5 | 2 (1–11) |
| O. warwicki | 17.4 | 6 (1–186) | 18 | 6 (1–186) | 16.5 | 7 (1–55) |
| D. tridens | 5.9 | 1 (1–7) | 7 | 1 (1–7) | 4.1 | 1.5 (1–3) |
Comparison of infestation state showed that the prevalence of infestation by each barnacle species was not significantly different between male and female crabs. Similarly, host sex had no impact on the median intensities of infestation by O. angulata and O. warwicki. On another hand, the median intensities of infestation by O. neptuni and D. tridens were determined by the host sex (Table 1).
Examined sample of female crabs included 74 ovigerous and 192 non-ovigerous individuals (Table 2). Prevalences of infestation by each of barnacle species of non-ovigerous crabs seemed to be higher than these of ovigerous crabs, but statistical analyses did not confirm these differences. Similarly, the median intensities of infestation did not differ significantly between these two host groups.
Table 2.
Prevalence (Prev, %), median and min, max of intensity (Md inten (Min–Max), barnacle individuals per host) of infestation by epibiotic barnacles for ovigerous and non-ovigerous Monomia haanii crabs
| Epibiont barnacles | Ovigerous crabs (N = 74) | Non-ovigerous crabs (N = 192) | ||
|---|---|---|---|---|
| Prev, % | Md inten (Min–Max) |
Prev, % | Md inten (Min–Max) |
|
| O. angulata | 43.2 | 4 (1–62) | 54.7 | 6 (1–92) |
| O. neptuni | 5.4 | 1 (1–2) | 8.3 | 2 (1–11) |
| O. warwicki | 13.5 | 17 (1–55) | 17.7 | 4 (1–24) |
| D. tridens | 2.7 | 2.5 (2–3) | 4.7 | 2 (1–3) |
The impact of the crab size on the infestation state is presented in Table 3. Crabs of less than 40 mm carapace width were not infested. The smallest M. haanii bearing stalked barnacles was the male with the carapace width of 41 mm. Crabs of the size group 40–49 mm were infested by 2 epibionts: O. angulata and O. warwicki. The smallest crabs infested by O. neptuni and D. tridens were found in the group 50–59 mm. Prevalence of infestation strongly positively correlated with crabs’ size (Table 4). Similarly, the positive correlations between carapace width of hosts and median intensities of infestation by distinct species were observed.
Table 3.
Prevalence (Prev, %), median and min, max of intensity (Md inten (Min–Max), barnacle individuals per host) of infestation by pedunculate barnacles on various-sized Monomia haanii
| Carapace width of crabs, mm | Number of crabs | O. angulata | O. neptuni | O. warwicki | D. tridens | ||||
|---|---|---|---|---|---|---|---|---|---|
| Prev, % | Md inten (Min–Max) |
Prev, % | Md inten (Min–Max) |
Prev, % | Md inten (Min–Max) |
Prev, % | Md inten (Min–Max) |
||
| <40 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40–49 | 41 | 34.2 | 3.5 (1–48) | 0 | 0 | 4.9 | 1 (1–1) | 0 | 0 |
| 50–59 | 108 | 36.1 | 2 (1–26) | 5.6 | 1 (1–2) | 9.3 | 7 (1–9) | 0.9 | 1 (1–1) |
| 60–69 | 229 | 44.1 | 3 (1–52) | 3.5 | 2 (1–3) | 8.3 | 2 (1–21) | 2.6 | 1.5 (1–2) |
| 70–79 | 233 | 62.2 | 9 (1–97) | 18.5 | 3 (1–36) | 27.9 | 6 (1–186) | 9.9 | 1 (1–7) |
| ≥80 | 74 | 60.8 | 12 (1–96) | 16.2 | 5.5 (1–32) | 33.8 | 9.5 (1–57) | 14.9 | 2 (1–2) |
Table 4.
Results of Spearman correlation test on the correlation between prevalence/median intensity of infestation by each barnacle species and the host size
| Barnacle species | Prevalence | Median intensity | ||
|---|---|---|---|---|
| pearson correlation | sig. | pearson correlation | sig. | |
| O. angulata | 0.930 | 0.007 | 0.903 | 0.014 |
| O. neptuni | 0.886 | 0.019 | 0.951 | 0.004 |
| O. warwicki | 0.935 | 0.006 | 0.811 | 0.050 |
| D. tridens | 0.908 | 0.012 | 0.901 | 0.014 |
Two species, O. angulata and O. neptuni colonized inner host branchial chambers at 4 different positions including: gills, maxillipeds, dorsal and ventral surfaces of the branchial chamber. O. warwicki attached to the external integument of the crab in the areas of the external surface of carapace, swimming legs, walking legs, chelipeds and abdomen. D. tridens were found on gills, the surface of the branchial chambers under the gills, maxillipeds and the carapace margin (the boundary between the branchial chamber and the external integument) (Table 5).
Table 5.
Distribution of pedunculate barnacles on Monomia haanii crabs
| Infested sites | Number of barnacles | |||
|---|---|---|---|---|
| O. angulata | O. neptuni | D. tridens | O. warwicki | |
| Gills | 2680 | 244 | 58 | 0 |
| Maxillipeds | 9 | 43 | 7 | 0 |
| Dorsal surface of the branchial chamber | 1438 | 6 | 0 | 0 |
| Ventral surface of the branchial chamber | 8 | 41 | 0 | 0 |
| Margin of carapace | 0 | 0 | 3 | 0 |
| External surface of carapace | 0 | 0 | 0 | 1399 |
| Swimming legs | 0 | 0 | 0 | 83 |
| Walking legs | 0 | 0 | 0 | 21 |
| Chelipeds | 0 | 0 | 0 | 10 |
| Abdomen | 0 | 0 | 0 | 3 |
| Total | 4135 | 334 | 68 | 1516 |
O. angulata: 4135 individuals of O. angulata were found on studied crabs with the majority attached on the surface of gills (64.8% of total) and the dorsal surface of the branchial chamber (34.8% of total) (Table 5). The median intensity of infestation differed significantly between gills 1 to 8. Most of O. angulata were concentrated on the surface of gills 4, 5 and 6. Among them, gill 4 was the most preferred site for the attachment with the largest number (1050 barnacles), the highest prevalence (30.4%) and the highest median intensity (3 (1–41)) of infestation. The infestation state parameters by each barnacle species on different gills are presented in Table 6.
Table 6.
Numer of barnacles (№), prevalence (Prev, %), median and min, max of intensity (Md inten (Min–Max), barnacle individuals per host) of infestation by epibiont barnacles on various gills of Monomia haanii crabs
| Gill number | O. angulata | O. neptuni | D. tridens | ||||||
|---|---|---|---|---|---|---|---|---|---|
| № | Prev, % | Md inten (Min–Max) |
№ | Prev, % | Md inten (Min–Max) |
№ | Prev, % | Md inten (Min–Max) |
|
| 1 | 2 | 0.3 | 1 (1–1) | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 0.1 | 1 | 1 | 0.1 | 1 | 0 | 0 | 0 |
| 3 | 80 | 6.5 | 1 (1–5) | 19 | 1.6 | 1 (1: 5) | 1 | 0.1 | 1 |
| 4 | 1050 | 30.4 | 3 (1–41) | 87 | 5.8 | 2 (1–8) | 38 | 3.9 | 1 (1–4) |
| 5 | 724 | 32 | 2 (1–22) | 64 | 3.5 | 2 (1–8) | 9 | 1.3 | 1 (1–1) |
| 6 | 575 | 25.2 | 2 (1–18) | 51 | 2.8 | 2 (1–11) | 8 | 0.9 | 1 (1–3) |
| 7 | 217 | 16.3 | 1 (1–8) | 22 | 1.7 | 1 (1–7) | 2 | 0.3 | 1 (1–1) |
| 8 | 31 | 3.0 | 1 (1–3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 2680 | 244 | 58 | ||||||
O. neptuni: Similarly to O. angulata, O. neptuni was found exclusively in the branchial chamber of the host, mostly on the gills (73.1% of the total, Table 5). Majority of these barnacles were found on the gills from 4 to 6 (82.8% of the total on gills, Table 6). The median intensities of infestation did not differ significantly between gills.
D. tridens: The surface of gills hosted 85.3% of the total number of D. tridens (58 individuals, Table 5). Only 3 individuals were found at the margin of the carapace of a single host. This species preferred to attach to gills from 3 to 7, gill 4 was found to bear up to 38 individuals of D. tridens (65.5% of the total on gills, Table 6). The statistical analysis did not show a significant difference in the intensity of infestation among gills.
O. warwicki: The vast majority of O. warwicki was recorded on the external surface of hosts’ carapaces (Table 5). The number of barnacles attached to swimming legs was higher than these on walking legs and chelipeds. A single large female crab was recorded bearing 3 individuals of this barnacle on its abdomen.
DISCUSSION
In the present study, the infestation of Monomia haanii crabs by epibiont pedunculate barnacles was reported for the first time. Three of these barnacles belong to genus Octolasmis, the remaining species is Dianajonesia tridens (previously treated as Temnaspis or Octolasmis tridens (Koçak, Kemal, 2008)). The studies on infestation by epibiotic barnacles conducted on 2 other common portunid crabs (Portunus pelagicus and P. sanguinolentus) in the same area showed that all 4 species infesting M. haanii infest these hosts as well (Oanh et al., 2018, 2018a). The wide distribution of O. angulata among hosts is acknowledged in other studies as well (Jeffries et al., 1982; Kumaravel et al., 2009; Li et al., 2014; Rasheed, Mustaquim, 2017). Also, in the study performed in the waters off Singapore the lack of host specialization was observed in O. warwicki and D. tridens, from 12 portunid crab species studied, they infested 10 and 7 species, respectively. However, only in Vietnamese waters all pedunculate barnacles infested all studied hosts without exception. Waters of Central Vietnam represent a region characterized by relatively low pedunculate barnacle species diversity, i.e. only 4 barnacle species were found settling on all 3 portunid crab hosts studied (Oanh et al., 2018, 2018a). In comparison, in the waters off Singapore 10 barnacle species infested portunid crabs (Jeffries et al., 1982), in Indian waters portunid crabs were observed hosting 6 species of barnacles (Kumaravel et al., 2009), in the waters off Karachi 5 barnacle species were infesting portunid crabs (Rasheed, Mustaquim, 2017). On another hand, prevalence of infestation in Vietnam, where it ranges from 50.8 to 84.5%, depending on the host is markedly higher than this parameter in Indian (11.1–35.3%) and Pakistan (2.9–10.8%) waters and is on par with the region off Singapore (12–100%, only the hosts where the studied sample size was more than 19 individuals were considered). Summing up, we tend to conclude that Vietnamese waters represent a region with a relatively low pedunculate species diversity, absence of host preference and a relatively high level of prevalence of infestation.
With the impact of the environmental conditions, the morphology or biology of the host itself could significantly influence on the barnacle infestation. In particular, in the waters adjacent to Singapore 56 decapod species were examined in order to assess the infestation by octolasmis barnacles (Jeffries et al., 1982). Twenty seven species (including 12 portunid crabs) of them were observed bearing barnacles. Infestation prevalence varied from 12 to 100% among these carbs. Similarly, in the waters off central Vietnam, prevalence of infestation of P. sanguinolentus (Oanh et al., 2018a) was quite high (84.5%), while this parameter reached 50.8% in P. pelagicus (Oanh et al., 2018) and 58.7% in M. haanii (present study). Thus, Monomia haanii falls into the group of crabs highly subjected for infestation but is still less infested than the most infested species such as P. sanguinolentus or Scylla serrata (Jeffries et al., 1982; Oanh et al., 2018a).
With the exception of O. neptuni and D. tridens, the infestation status expressed in both prevalence and intensity did not depend on the sex of M. haanii. The same pattern of host sex dependent distribution was observed in barnacles infesting P. sanguinolentus in the waters off Vietnam (Oanh et al., 2018a). On the contrary, study on P. pelagicus in the same area revealed that host’s sex impact the abundance of all pedunculate barnacles, except O. neptuni (Binh et al., 2018). This observation leads us to conclusion that impact of the host’s sex is random and does not impact the infestation by pedunculate barnacles by itself. Similarly, studies in other regions do not reveal any clear pattern in impact of the host’s sex on infestation. Some studies show that there is no connection between these parameters (Santos, Bueno, 2002; Li et al., 2014), while in other prevalence, intensity or both parameters were significantly different between male and female hosts, even if the same species of the host or barnacle were studied (Rasheed and Mustaquim, 2017; Machado et al., 2013; Sheilds, 1992).
Infestation by stalked barnacles strongly positively correlated with the carapace width of the host. The similar relationship was reported before on some other host species such as O. angulata on Scylla serrata (Jeffries et al., 1992), O. lowei on Callinectes ornatus (Santos, Bueno, 2002), O. bullata on Portunus sanguinolentus (Li et al., 2014), O. angulata and D. tridens on P. sanguinolentus (Oanh et al., 2018a). The previous studies demonstrated that the larger crabs have the longer duration of the intermolt phrase (Jeffries et al., 1992; Josileen, Menon, 2005). This explains the correlation between infestation state and size of host crab because the longer interval between two ecdyses of host allows a greater amount of epibionts to settle.
Epibiotic barnacles did not distribute on the host’s body randomly. O. angulata and O. neptuni were observed only in branchial chambers, O. warwicki was the only species which settled on the external integument of M. haanii, while D. tridens occurred both in the branchial chambers and at the host carapace’s margin. These records are consistent with previous reports on distribution sites of these barnacles infesting other hosts, such as P. pelagicus (Oanh et al., 2018), P. sanguinolentus (Oanh et al., 2018a), Scylla serrata and Thalamita danae (Jeffries et al., 2005). Voris and Jeffries (1997) hypothesized that the barnacles living inside the branchial chambers of decapods, where they covered and protected by host stabilized carapace (such as O. bullata, O. angulata, O. neptuni, O. lowei…) possess small, delicate capitular plates. Others with strong, large capitular plates tend to attach themselves to the external surface of the host (Voris, Jeffries, 1997). Our results generally match with these hypotheses, in particular, O. warwicki and D. tridens having the largest capitular plates among studied species tend to settle on the external integument of the crab or the carapace margin, while O. angulata and O. neptuni, which have small capitular plates, settle exclusively in the branchial chambers. However, this general trend is broken in D. tridens, which tends to settle on the gills. According to Voris and Jeffries (1997) this observation may be explained by the tendency of this barnacle to settle in the areas where they are more exposed to the pressure of respiratory currents, but our observation does not support this hypothesis. The explanation for the concentration of barnacles in the branchial chambers was given in the articles published earlier (Jeffries, Voris, 1983; Gannon, Wheatly, 1992; Voris et al., 2000; Li et al., 2014). In particular, the host’s respiratory currents, supplying food and oxygen for permanently fixed epibionts, may determine their settlement positions. Our findings completely correspond to this theory, in particular, gills were the most occupied part of the branchial chamber (Table 5). In turn, gill 4, which is exposed to the strongest respiratory currents (Arudpragasam, Naylor, 1964) was the most preferred attachment site.
CONCLUSIONS
The present study confirms some trends observed in preceding research. Vietnamese waters appear to be a region characterised by relatively low diversity of pedunculate barnacles and relatively high intensity of infestation. None of the barnacle species inhabiting the area of research was host specific, which also can be considered the specific trait of this area.
Portunid crab Monomia haanii seems to be highly subjected to barnacle infestation, additionally the crab’s size has a significant impact on the prevalence of infestation. On another hand no relationship between crab’s sex and infestation state was observed.
Finally the distribution of the pedunculate barnacles on the host’s body generally followed the basic distributional patterns of this group of epibionts.
Список литературы
Arudpragasam K.D., Naylor E., 1964. Gill ventilation and the role of resersed respiratory currents in Carcinus maenas (L.) // Journal of Experimental Biology. V. 41. P. 299–307.
Binh D.T., Oanh L.T.K., Sang T.Q., Oanh T.T., Glenner H., 2018. Species diversity and phylogenetic relationships of symbiotic crustaceans on Portunus pelagicus (Linnaeus, 1758) in Vietnam. Available from: https://www.researchgate.net (accessed 15 August 2020)
Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W., 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited // Journal of Parasitology. V. 83. P. 575583. https://doi.org/10.2307/3284227
Chertoprud E.S., Spiridinov V.A., Ponomarev S.A., Mokievsky V.C., 2012. Commercial crabs (Crustacea Decapoda Brachyura) from Nhatrang Bay (Vietnam) // Benthic fauna of the Bay of Nhatrang, Southern Vietnam. Britayev T.A. and Pavlov D.S. (Eds). Moscow: KMK Scientific Press Ltd. V. 2. P. 296–345.
Gannon A.T., Wheatly M.G., 1992. Physiological effects of an ectocommensal gill barnacle, Octolasmis muelleri, on gas exchange in the blue crab Callinectes sapidus // Journal of Crustacean Biology. V. 12. P. 11–18. https://doi.org/10.2307/1548714
Hassan M., Aziz M.F.H.A., Kismiyati K., Subekti S., Zakariah M.I., 2019. Occurrence of pedunculate barnacle, Octolasmis spp. in blue swimming crab, Portunus pelagicus // Scientific Journal of Fisheries and Marine Sciences. V. 11. P. 1–8. https://doi.org/10.20473/jipk.v11i1.10635
Hudson D.A., Lester R.J.G., 1994. Parasites and symbionts of wild mud crabs Scylla serrata (Forskal) of potential significance in aquaculture // Aquaculture. V. 120. P. 183–199. https://doi.org/10.1016/0044-8486(94)90077-9
Jeffries W.B., Voris H.K., Yang C.M., 1982. Diversity and distribution of the pedunculate barnacle Octolasmis in the seas adjacent to Singapore // Journal of Crustacean Biology. V. 2. P. 562–569. https://doi.org/10.2307/1548096
Jeffries W.B., Voris H.K., 1983. The distribution, size, and reproduction of the pedunculate barnacle, Octolasmis muelleri (Coker, 1902), on the blue crab, Callinectes sapidus (Rathbun, 1896) // Fieldiana Zoology. V. 16. P. 1–10. https://doi.org/10.5962/bhl.title.3097
Jeffries W.B., Voris H.K., Yang C.M., 1989. A new mechanism of host colonization: Pedunculate barnacles of the genus Octolasmis on the mangrove crab Scylla serrata // Ophelia. V. 31. P. 51–58. https://doi.org/10.1080/00785326.1989.10430850
Jeffries W.B., Voris H.K., Poocachiranon S., 1992. Age of the mangrove crab Scylla serrata at colonization by stalked barnacles of the genus Octolasmis // The Biological Bulletin. V. 182. P. 188–194. https://doi.org/10.2307/1542112
Jeffries W.B., Voris H.K., Naiyanetr P., Panha S., 2005. Pedunculate barnacles of the symbiotic genus Octolasmis (Cirripedia: Thoracica: Poecilasmatidae) from the Northern Gulf of Thailand // Natural History Journal of Chulalongkorn University. V. 5. P. 9–13.
Jeffries W.B., Voris H.K., 2005. Crustacean hosts of the pedunculate barnacle genus Octolasmis in the northern Gulf of Mexico // Gulf of Mexico Science. V. 22. P. 173–188. https://doi.org/10.18785/goms.2202.05
Josileen J., Menon N.G., 2005. Growth of the blue swimmer crab, Portunus pelagicus in captivity // The Natural History Journal of Chulalongkorn University. V. 78. P. 1–18. https://doi.org/10.1163/1568540054024556
Koçak A., Kemal M., 2008. Notes on the nomenclature of some genus group names in Arthropoda // Miscellaneous Papers, Centre for Entomological Studies, Ankara. P. 138.
Kumaravel K., Ravichandran S., Rameshkumar G., 2009. Distribution of barnacle Octolasmis on the gill region of some edible crabs // Academic Journal of Entomology. V. 2. P. 36–39.
Li H.X., Ma L.S., Yan Y., Yang C.P., Lin C.X., 2014. First records of the epizoic barnacle Octolasmis bullata (Cirripedia: Thoracia: Poecilasmatidae) on the swimming crab Portunus sanguinolentus (Decapoda: Portunidae) // Journal of Crustacean Biology. V. 34. P. 76–81. https://doi.org/10.1163/1937240X-00002209
Machado G.B.O, Sanches F.H.C., Fortuna M.D., Costa T.M., 2013. Epibiosis in decapod crustaceans by stalked barnacle Octolasmis lowei (Cirripedia: Poecilasmatidae) // Zoologia. V. 30. P. 307–311. https://doi.org/10.1590/S1984-46702013000300007
Oanh L.T.K., Sang T.Q., Binh D.T., 2018. Infestation status of pedunculate barnacle (Octolsamis spp.) in blue swimming crab (Portunus pelagicus, Linnaeus 1758) in Khanh Hoa // Journal of Malaria and Parasite Diseases Control. V. 104. P. 60–66.
Oanh L.T.K., Ha V.T., Thanh N.T.H., 2018a. Some ectoparasites on three-spot swimming crab Portunus sanguinolentus, Herbst 1783) in Khanh Hoa province // Journal of Tropical Science and Technology. V. 17. P. 28–38.
Rasheed S., Mustaquim H., 2017. Occurrence of pedunculate barnacles of the symbiotic genus Octolasmis (Cirripedia: Crustacea) in two species of edible crabs // Pakistan Journal of Zoology. V. 49. P. 1879–1888. https://doi.org/10.17582/journal.pjz/2017.49.5.1879.1888
Santos C., Bueno S.L.S., 2002. Infestation by Octolasmis lowei (Cirripedia: Poecilasmatidae) in Callinectes danae and Callinectes ornatus (Decapoda: Portunidae) from São Sebastião // Journal of Crustacean Biology. V. 22. P. 241–248. https://doi.org/10.1163/20021975-99990231
Shields J.D., 1992. Parasites and symbionts of the crab Portunus pelagicus from Moreton Bay, Eastern Australia // Journal of Crustacean Biology. V. 12. P. 94–100. https://doi.org/10.2307/1548723
Voris H.K., Jeffries W.B., 1997. Size, distribution, and significance of capitular plates in Octolasmis (Cirripedia: Poecilasmatidae) // Journal of Crustacean Biology. V. 17. P. 217–226. https://doi.org/10.2307/1549272
Voris H.K., Jeffries W.B., Poovachiranon S., 2000. Size and location of stalked barnacles of the genus Octolasmis on the mangrove crab Scylla serrata // Journal of Crustacean Biology. V. 20. P. 483–494. https://doi.org/10.1163/20021975-99990064
Windsor A.M., Mendoza J.C.E., Deeds J.R., 2019. Resolution of the Portunus gladiator species complex: taxonomic status and identity of Monomia gladiator (Fabricius, 1798) and Monomia haanii (Stimpson, 1858) (Brachyura, Decapoda, Portunidae) // ZooKeys. V. 858. P. 11–43. https://doi.org/10.3897/zookeys.858.33826
Дополнительные материалы отсутствуют.
Инструменты
Зоологический журнал